Answered step by step
Verified Expert Solution
Question
1 Approved Answer
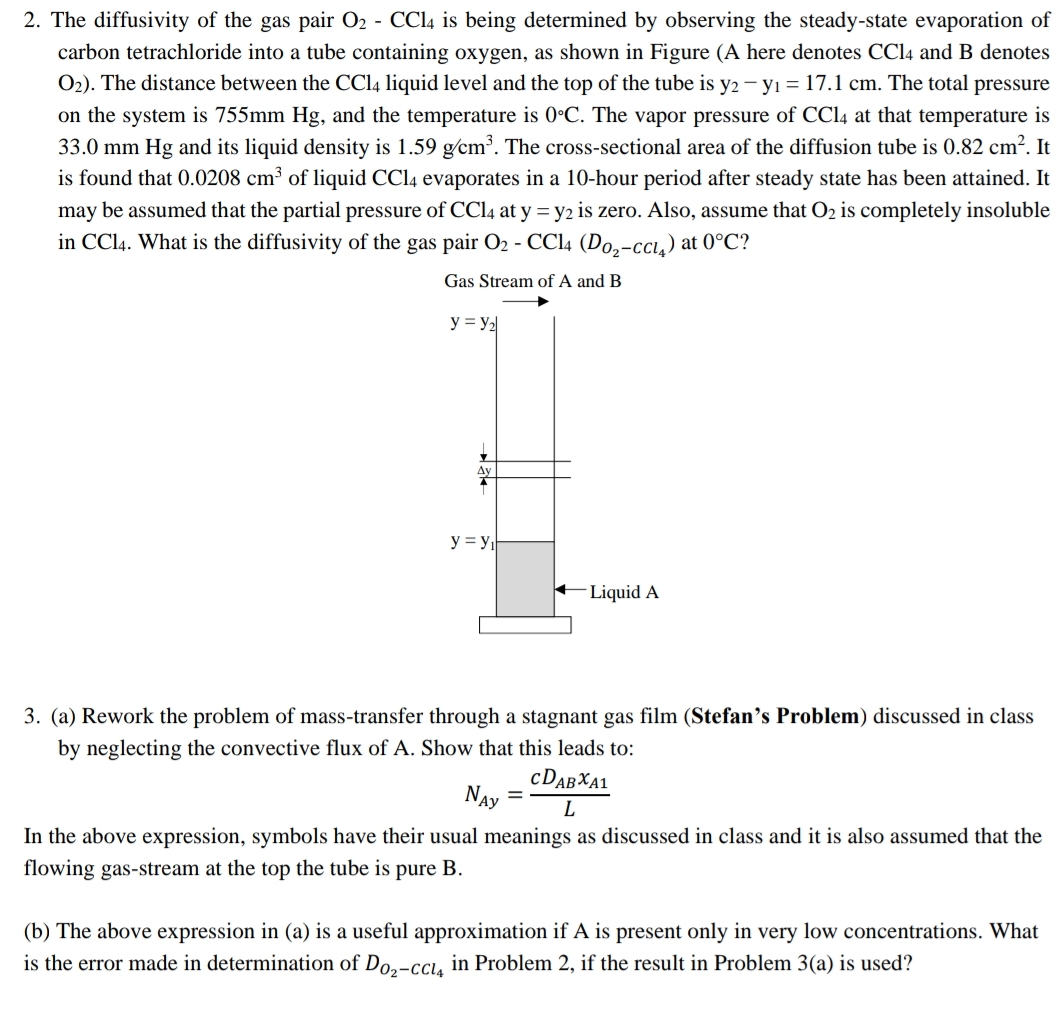
The diffusivity of the gas pair O 2 - C C l 4 is being determined by observing the steady - state evaporation of carbon
The diffusivity of the gas pair is being determined by observing the steadystate evaporation of carbon tetrachloride into a tube containing oxygen, as shown in Figure A here denotes and denotes The distance between the liquid level and the top of the tube is The total pressure on the system is and the temperature is The vapor pressure of at that temperature is and its liquid density is The crosssectional area of the diffusion tube is It is found that of liquid evaporates in a hour period after steady state has been attained. It may be assumed that the partial pressure of at is zero. Also, assume that is completely insoluble in What is the diffusivity of the gas pair at
Gas Stream of A and B
a Rework the problem of masstransfer through a stagnant gas film Stefans Problem discussed in class by neglecting the convective flux of A Show that this leads to:
In the above expression, symbols have their usual meanings as discussed in class and it is also assumed that the flowing gasstream at the top the tube is pure B
b The above expression in a is a useful approximation if A is present only in very low concentrations. What is the error made in determination of in Problem if the result in Problem a is used?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


