Answered step by step
Verified Expert Solution
Question
1 Approved Answer
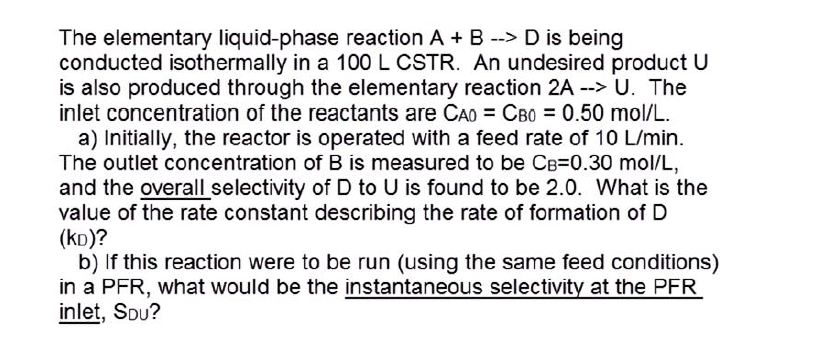
The elementary liquid - phase reaction A + B - - > D is being conducted isothermally in a 1 0 0 L CSTR .
The elementary liquidphase reaction A B is being
conducted isothermally in a CSTR An undesired product
is also produced through the elementary reaction The
inlet concentration of the reactants are
a Initially, the reactor is operated with a feed rate of
The outlet concentration of is measured to be
and the overall selectivity of to is found to be What is the
value of the rate constant describing the rate of formation of
b If this reaction were to be run using the same feed conditions
in a PFR what would be the instantaneous selectivity at the PFR
inlet, Sou?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


