Answered step by step
Verified Expert Solution
Question
1 Approved Answer
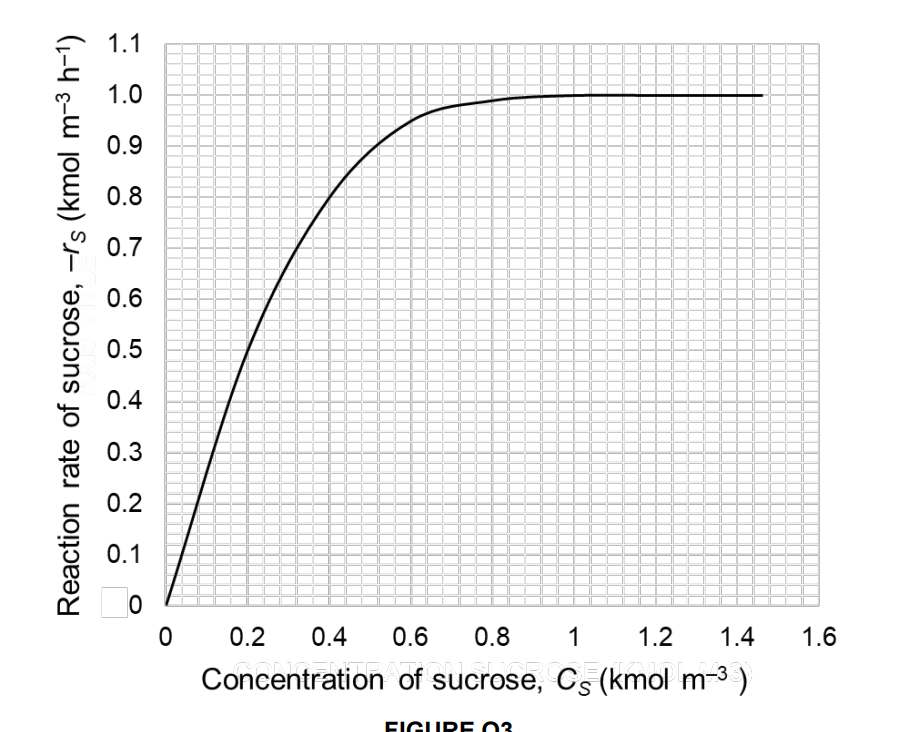
The enzyme Invertase is used as a catalyst for the inversion of sucrose, a very important process in the food industry. To study this reaction,
The enzyme Invertase is used as a catalyst for the inversion of sucrose, a very
important process in the food industry. To study this reaction, we introduce L of a solution containing kg
of sucrose and kg of Invertase, in a jacketed stirred tank reactor. The density of the solution remains constant
throughout the entire process.
b Under normal operational conditions, the process will be stopped as soon as the reaction rate is half of its initia
value, and it will require hour to empty the reactor, sterilise it and prepare it for the next batch. Calculate the
number of cycles that can be completed per day. Consider the molecular weight of sucrose to be kgkmolConcentration of sucrose,
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


