Answered step by step
Verified Expert Solution
Question
1 Approved Answer
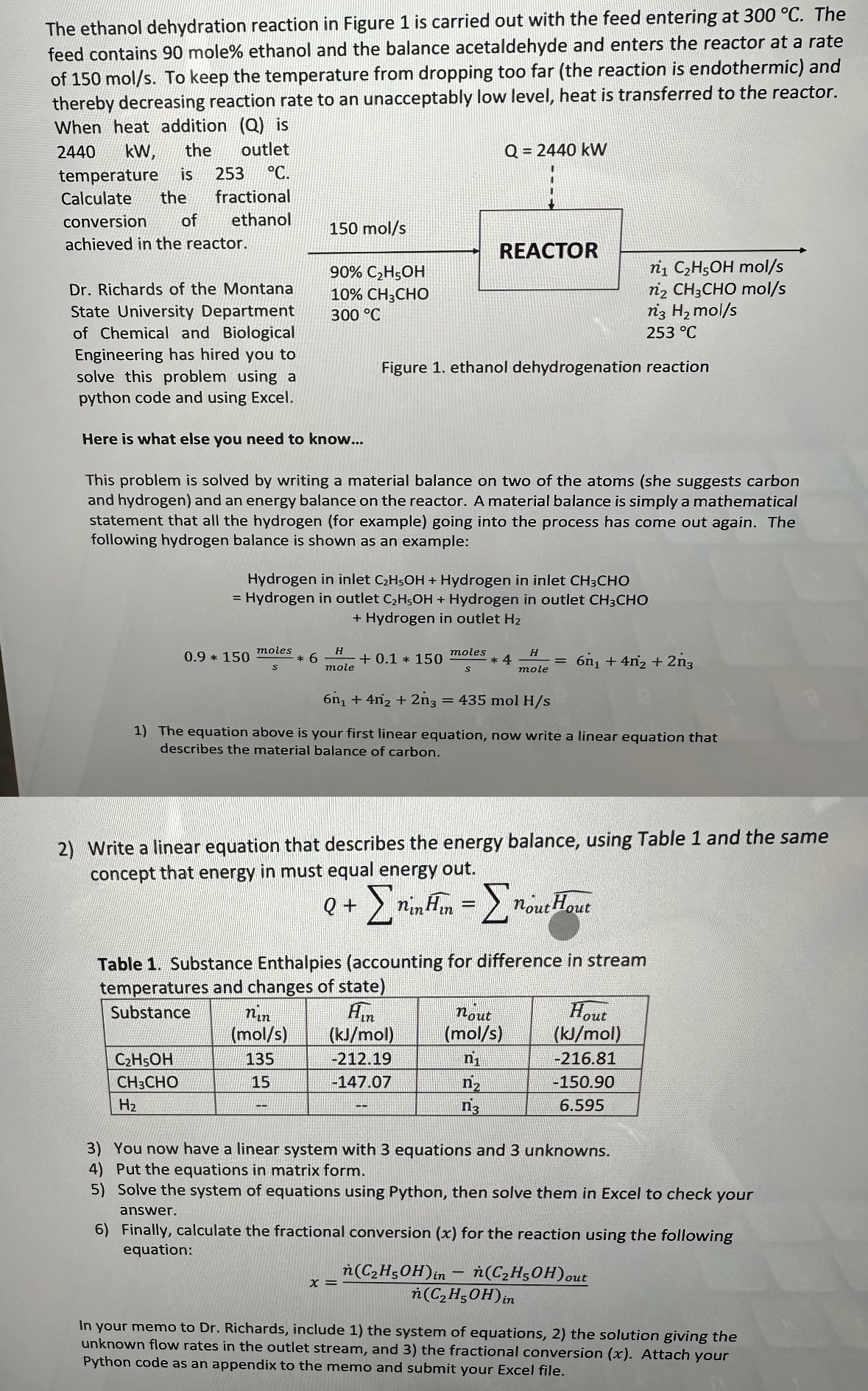
The ethanol dehydration reaction in Figure 1 is carried out with the feed entering at 3 0 0 C . The feed contains 9 0
The ethanol dehydration reaction in Figure is carried out with the feed entering at The feed contains mole ethanol and the balance acetaldehyde and enters the reactor at a rate of To keep the temperature from dropping too far the reaction is endothermic and thereby decreasing reaction rate to an unacceptably low level, heat is transferred to the reactor. When heat addition is the outlet temperature is Calculate the fractional conversion of ethanol achieved in the reactor.
Dr Richards of the Montana State University Department of Chemical and Biological Engineering has hired you to solve this problem using a python code and using Excel.
Here is what else you need to know...
This problem is solved by writing a material balance on two of the atoms she suggests carbon and hydrogen and an energy balance on the reactor. A material balance is simply a mathematical statement that all the hydrogen for example going into the process has come out again. The following hydrogen balance is shown as an example:
Hydrogen in inlet Hydrogen in inlet CHO
Hydrogen in outlet Hydrogen in outlet CHO
Hydrogen in outlet
mol
The equation above is your first linear equation, now write a linear equation that describes the material balance of carbon.
Write a linear equation that describes the energy balance, using Table and the same concept that energy in must equal energy out.
widehatwidehat
Table Substance Enthalpies accounting for difference in stream temperatures and changes of state
tableSubstancetable
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


