Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The ethanol-water solution containing 42% ethanol as a weight percent is fed to a distillation column operating at atmospheric pressure, and it is desired to
The ethanol-water solution containing 42% ethanol as a weight percent is fed to a distillation column operating at atmospheric pressure, and it is desired to separate into a head product containing 90% ethanol and a base product containing 90% water. The enthalpy of the feed stream entering the column as a vapor-liquid mixture is 460.5 kJ/kg. An entire condenser is connected to the column, and the liquid phase enthalpy leaving the condenser is 117.2 kJ/kg. Saturated water vapor at a pressure of 14 kg/cm2 (1373 kPa) is sent from the bottom shelf of the column, 0.416 kg per kilogram of feed stream. The enthalpy of saturated water vapor at this pressure is 2793 kJ/kg. Using the Ponchon-Savarit method;
a) Find what the Riflax ratio should be.
b) Find the theoretical number of stages and the location of the feeding rack.
c) Distillation process using the entire evaporator and at the calculated riflux rate
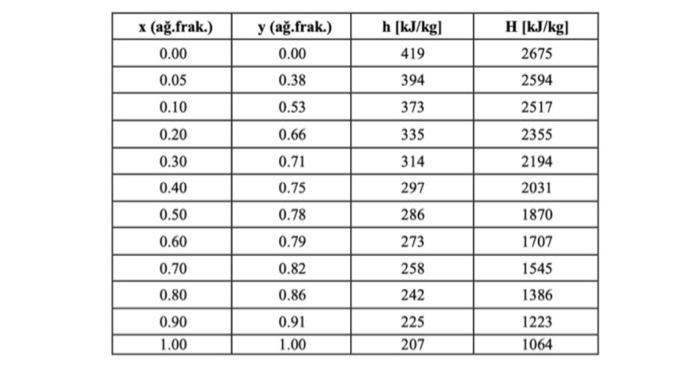
Calculate the minimum number of racks and the minimum reflux ratio. Equilibrium and enthalpy values of the ethanol-water system (at 101.3 kPa pressure)
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


