Question
The feed to a flash drum consists of 25 mol% n-butane (1), 35% isobutene (2), 30% n-pentane (3), and 10% n-hexane (4). This mixture can
The feed to a flash drum consists of 25 mol% n-butane (1), 35% isobutene (2), 30% n-pentane (3), and 10% n-hexane (4). This mixture can be assumed to form an ideal mixture in both liquid and vapor phase.
a) Calculate the range of pressures for which the above mixture will be in two phases at 289 K.
b) Calculate the range of temperatures for which the above mixture will be in two phases at 1 bar.
c) Suppose 100 kmol/h of the feed is introduced into a flash drum held at 289 K and 1 bar. Determine the compositions and molar flow rates of the vapor and liquid products.
d) Suppose 100 kmol/h of the feed is introduced into a flash drum held at 289 K. If the vapor stream leaving the drum has a flow rate of 50 kmol/h, determine the drum pressure as well as the compositions of the liquid and vapor products.
e) Suppose 100 kmol/h of the feed is introduced into a flash drum held at 1 bar. It is required to get a liquid stream with composition of 12.6% n- butane, 12.6% isobutene, 44.2% n-pentane, and 30.6% n-hexane. Determine the temperature and flow rates of the vapor and liquid stream leaving the flash drum.
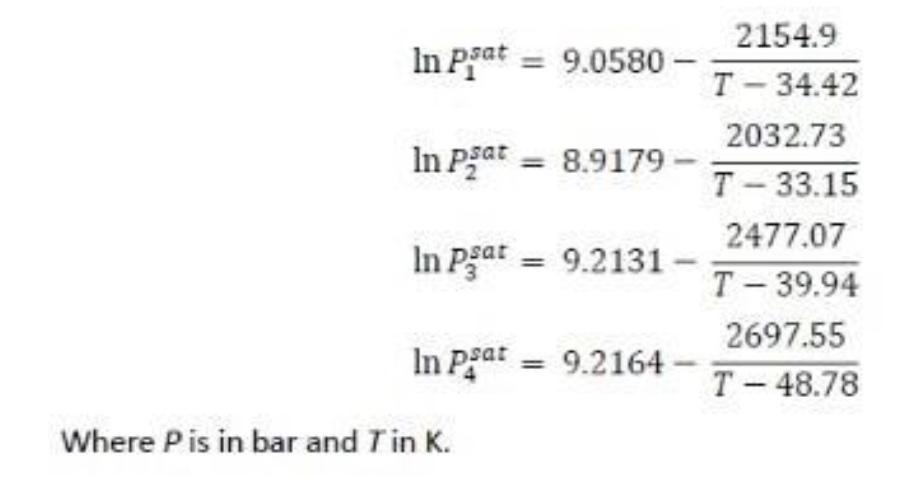
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


