 The following are the previous parts to the questions which have been answered. I just need the answers and explanations for question 3.
The following are the previous parts to the questions which have been answered. I just need the answers and explanations for question 3.
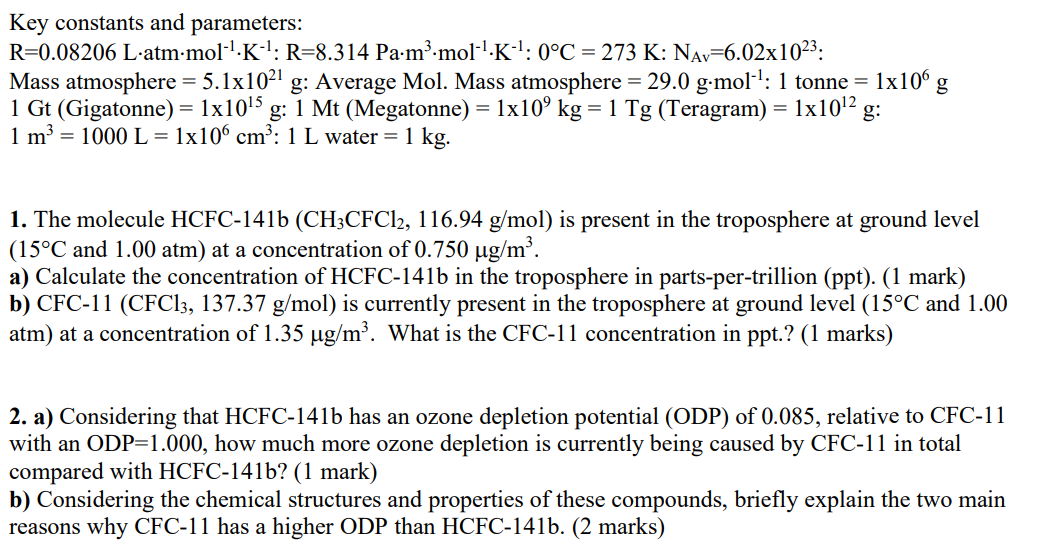
The following are the answers to questions 1 and 2:



3. HCFC-141b has a rate constant for reaction with hydroxyl radical of 5.11015cm3molec1s1 (at 15C ). a) From the information in 1., what is the concentration of HCFC-141b in molec. cm3?(1mark) b) Given the rate constant above and a hydroxyl radical concentration of 4.0105moleccm3, estimate the rate of reaction of HCFC-141b with hydroxyl radical (in molec cm3s1).(2 marks) c) If the entire atmosphere was at 15C and 1atm pressure, what would be the total mass of HCFC 141b removed from the atmosphere by hydroxyl reaction in a year (in tonnes)? (2 marks) Key constants and parameters: R=0.08206Latmmol1K1:R=8.314Pam3mol1K1:0C=273K:NAv=6.02x1023 : Mass atmosphere =5.11021g : Average Mol. Mass atmosphere =29.0gmol1:1 tonne =1106g 1Gt( Gigatonne )=11015g:1Mt (Megatonne) =1109kg=1Tg( Teragram )=11012g : 1m3=1000L=1106cm3:1L water =1kg. 1. The molecule HCFC-141b (CH3CFCl2,116.94g/mol) is present in the troposphere at ground level (15C and 1.00atm) at a concentration of 0.750g/m3. a) Calculate the concentration of HCFC-141b in the troposphere in parts-per-trillion (ppt). (1 mark) b) CFC-11 (CFCl3,137.37g/mol) is currently present in the troposphere at ground level (15C and 1.00 atm) at a concentration of 1.35g/m3. What is the CFC-11 concentration in ppt.? (1 marks) 2. a) Considering that HCFC-141b has an ozone depletion potential (ODP) of 0.085, relative to CFC-11 with an ODP=1.000, how much more ozone depletion is currently being caused by CFC-11 in total compared with HCFC-141b? (1 mark) b) Considering the chemical structures and properties of these compounds, briefly explain the two main reasons why CFC-11 has a higher ODP than HCFC-141b. (2 marks) Concentration of HCFC141b in glm3=0.750g/m3 Conceutration of HCFC-141b in PPT =? As, DPP =Lng So, Concentration of HCFC141b in PPt, So, HCFC-14)b in pPt =750000ppt or 7.50105Ppt. Concentration of CFC-11 in Hg/m3=1.35g/m3 As, PPt =ng/L Concentration of CFC11 in ppt, =1.35g/m3(1g109ng)(1000dm31m3)(1L1dm3)=1.35gtm106ng/L=1.35106ppt Hence, CFC-11 in ppt =1.35106 ppt Answer a Given that, Ozone depletion Potential (ODP) of HCFC- 1416 is 0.085 times of CFC-11, So, suppose that, ting lppt of HCFC14i6 causes ODP=0.085 Ippt of CFC-11 Causes ODP=1.000 So, ODP Caused by CFC11=0.0851.0007.50105PPt1.35106PPt=21.18 Hence, =21.187.50105PPt CFC11 is causing ODP21.18 times more than 2, (b) We know that ozone depletion is mainly Caused by chloride radicals produced from CFC, and HCFCs. Looking at the structures, HCFC 1416 has fwo chlorine atoms while in CFC-11, there are three chlorine atoms. This means that CFC-11 produces more chloride radicals than HCFC-141b and hence causes more ozone depletion. 3. HCFC-141b has a rate constant for reaction with hydroxyl radical of 5.11015cm3molec1s1 (at 15C ). a) From the information in 1., what is the concentration of HCFC-141b in molec. cm3?(1mark) b) Given the rate constant above and a hydroxyl radical concentration of 4.0105moleccm3, estimate the rate of reaction of HCFC-141b with hydroxyl radical (in molec cm3s1).(2 marks) c) If the entire atmosphere was at 15C and 1atm pressure, what would be the total mass of HCFC 141b removed from the atmosphere by hydroxyl reaction in a year (in tonnes)? (2 marks) Key constants and parameters: R=0.08206Latmmol1K1:R=8.314Pam3mol1K1:0C=273K:NAv=6.02x1023 : Mass atmosphere =5.11021g : Average Mol. Mass atmosphere =29.0gmol1:1 tonne =1106g 1Gt( Gigatonne )=11015g:1Mt (Megatonne) =1109kg=1Tg( Teragram )=11012g : 1m3=1000L=1106cm3:1L water =1kg. 1. The molecule HCFC-141b (CH3CFCl2,116.94g/mol) is present in the troposphere at ground level (15C and 1.00atm) at a concentration of 0.750g/m3. a) Calculate the concentration of HCFC-141b in the troposphere in parts-per-trillion (ppt). (1 mark) b) CFC-11 (CFCl3,137.37g/mol) is currently present in the troposphere at ground level (15C and 1.00 atm) at a concentration of 1.35g/m3. What is the CFC-11 concentration in ppt.? (1 marks) 2. a) Considering that HCFC-141b has an ozone depletion potential (ODP) of 0.085, relative to CFC-11 with an ODP=1.000, how much more ozone depletion is currently being caused by CFC-11 in total compared with HCFC-141b? (1 mark) b) Considering the chemical structures and properties of these compounds, briefly explain the two main reasons why CFC-11 has a higher ODP than HCFC-141b. (2 marks) Concentration of HCFC141b in glm3=0.750g/m3 Conceutration of HCFC-141b in PPT =? As, DPP =Lng So, Concentration of HCFC141b in PPt, So, HCFC-14)b in pPt =750000ppt or 7.50105Ppt. Concentration of CFC-11 in Hg/m3=1.35g/m3 As, PPt =ng/L Concentration of CFC11 in ppt, =1.35g/m3(1g109ng)(1000dm31m3)(1L1dm3)=1.35gtm106ng/L=1.35106ppt Hence, CFC-11 in ppt =1.35106 ppt Answer a Given that, Ozone depletion Potential (ODP) of HCFC- 1416 is 0.085 times of CFC-11, So, suppose that, ting lppt of HCFC14i6 causes ODP=0.085 Ippt of CFC-11 Causes ODP=1.000 So, ODP Caused by CFC11=0.0851.0007.50105PPt1.35106PPt=21.18 Hence, =21.187.50105PPt CFC11 is causing ODP21.18 times more than 2, (b) We know that ozone depletion is mainly Caused by chloride radicals produced from CFC, and HCFCs. Looking at the structures, HCFC 1416 has fwo chlorine atoms while in CFC-11, there are three chlorine atoms. This means that CFC-11 produces more chloride radicals than HCFC-141b and hence causes more ozone depletion
 The following are the previous parts to the questions which have been answered. I just need the answers and explanations for question 3.
The following are the previous parts to the questions which have been answered. I just need the answers and explanations for question 3.









