Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The following data are for the decomposition of dinitrogen pentoxide in carbon tetrachloride solution at 30 C. NO5 2 NO+2 0 NO5 ], M
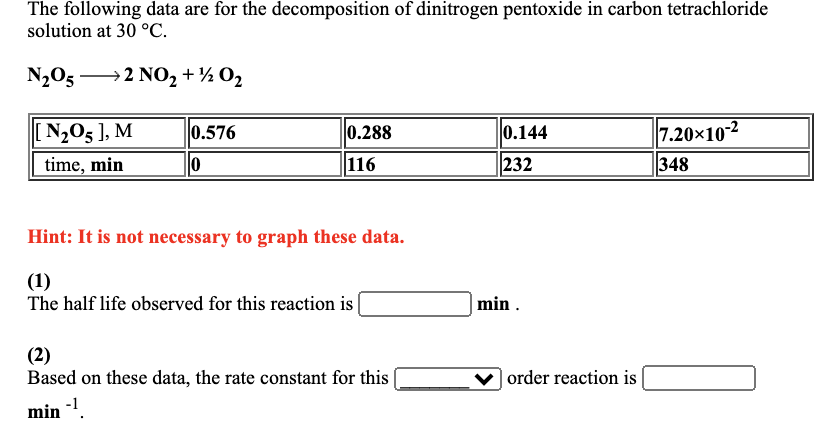
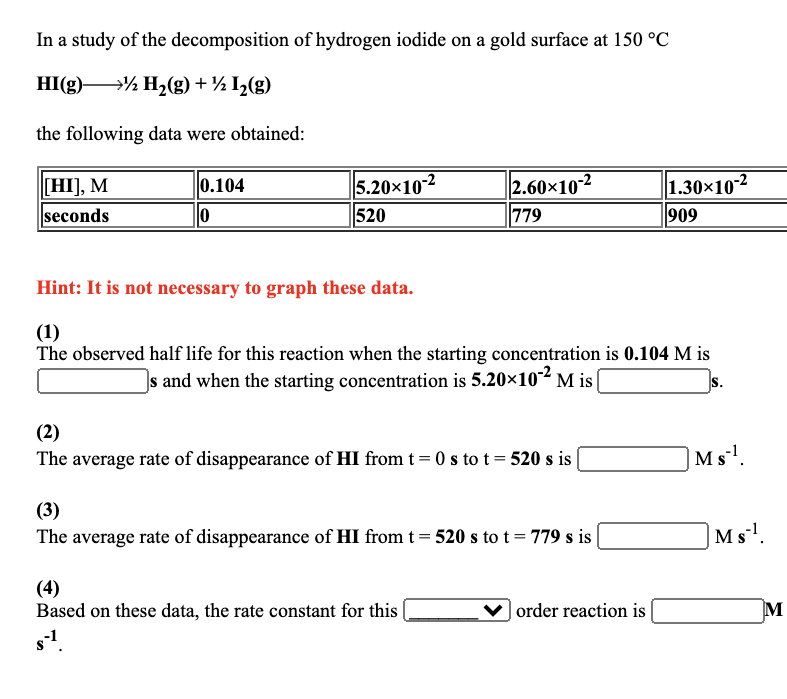
The following data are for the decomposition of dinitrogen pentoxide in carbon tetrachloride solution at 30 C. NO5 2 NO+2 0 NO5 ], M time, min 0.576 0 0.288 116 Hint: It is not necessary to graph these data. (1) The half life observed for this reaction is (2) Based on these data, the rate constant for this min - 0.144 232 min. order reaction is 7.2010-2 348 In a study of the decomposition of hydrogen iodide on a gold surface at 150 C HI(g) H(g) + 1(g) the following data were obtained: [HI], M seconds 0.104 0 5.2010-2 520 2.6010-2 779 Hint: It is not necessary to graph these data. (1) The observed half life for this reaction when the starting concentration is 0.104 M is s and when the starting concentration is 5.2010-2 M is S. (2) The average rate of disappearance of HI from t=0 s to t = 520 s is (3) The average rate of disappearance of HI from t = 520 s to t = 779 s is (4) Based on these data, the rate constant for this s-. 1.30-10-2 909 order reaction is Ms. Ms. M
Step by Step Solution
★★★★★
3.44 Rating (144 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


