Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The following data was collected for the titration of 0.2M KOH with an unknown molarity of HCI. Calculate the volume used in each titration
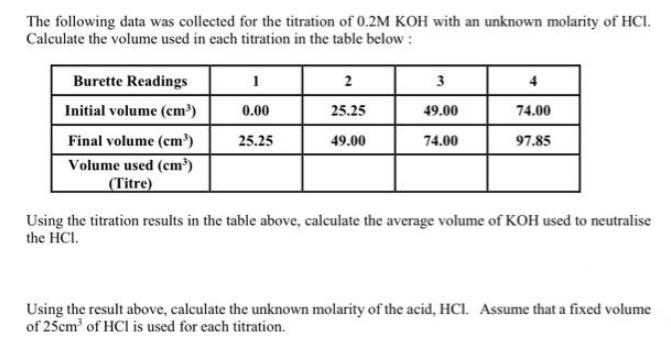
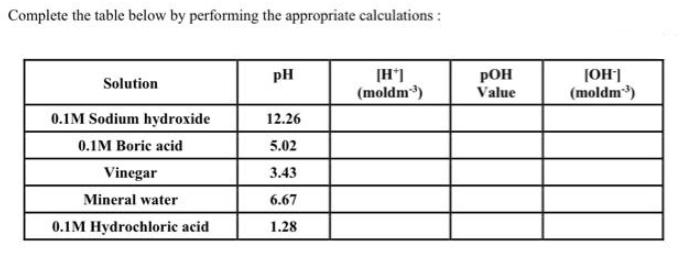
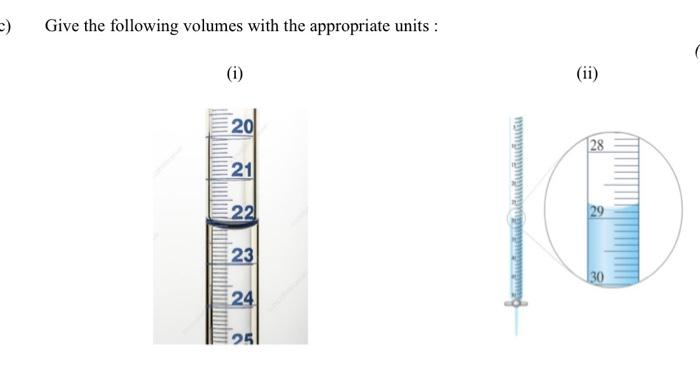
The following data was collected for the titration of 0.2M KOH with an unknown molarity of HCI. Calculate the volume used in each titration in the table below: Burette Readings Initial volume (cm) Final volume (cm) Volume used (cm) (Titre) 1 0.00 25.25 2 25.25 49.00 3 49.00 74.00 4 74.00 97.85 Using the titration results in the table above, calculate the average volume of KOH used to neutralise the HCI. Using the result above, calculate the unknown molarity of the acid, HCI. Assume that a fixed volume of 25cm of HCl is used for each titration. Complete the table below by performing the appropriate calculations: Solution 0.1M Sodium hydroxide 0.1M Boric acid Vinegar Mineral water 0.1M Hydrochloric acid PH 12.26 5.02 3.43 6.67 1.28 [H*] (moldm) POH Value [OH-] (moldm) Give the following volumes with the appropriate units: (i) 20 21 22 23 24 25 (ii) 28
Step by Step Solution
★★★★★
3.44 Rating (151 Votes )
There are 3 Steps involved in it
Step: 1
To determine the average volume of KOH used compute the amount utilized in each titration using the ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


