Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The following reaction equation represents the burning of Octane C8H18 in oxygen O2 : xC8H18+yO2zCO2+wHO2 where CO2 represents carbon dioxide. a) Deduce the linear system
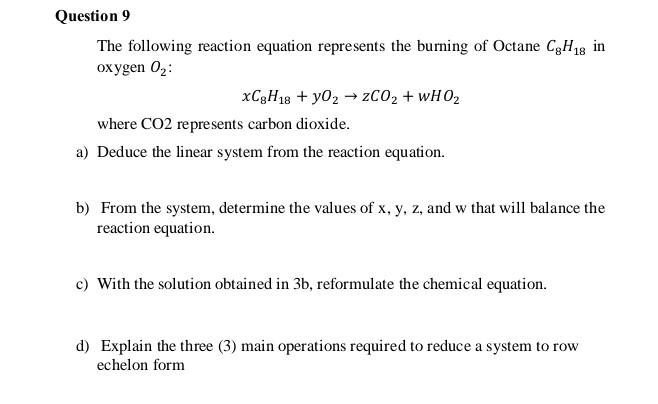
The following reaction equation represents the burning of Octane C8H18 in oxygen O2 : xC8H18+yO2zCO2+wHO2 where CO2 represents carbon dioxide. a) Deduce the linear system from the reaction equation. b) From the system, determine the values of x,y,z, and w that will balance the reaction equation. c) With the solution obtained in 3b, reformulate the chemical equation. d) Explain the three (3) main operations required to reduce a system to row echelon form The following reaction equation represents the burning of Octane C8H18 in oxygen O2 : xC8H18+yO2zCO2+wHO2 where CO2 represents carbon dioxide. a) Deduce the linear system from the reaction equation. b) From the system, determine the values of x,y,z, and w that will balance the reaction equation. c) With the solution obtained in 3b, reformulate the chemical equation. d) Explain the three (3) main operations required to reduce a system to row echelon form
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


