Question
The following reactions take place in series. The first is Oth order. The second is 1st order. Reaction 1: A D Reaction 2: D-U
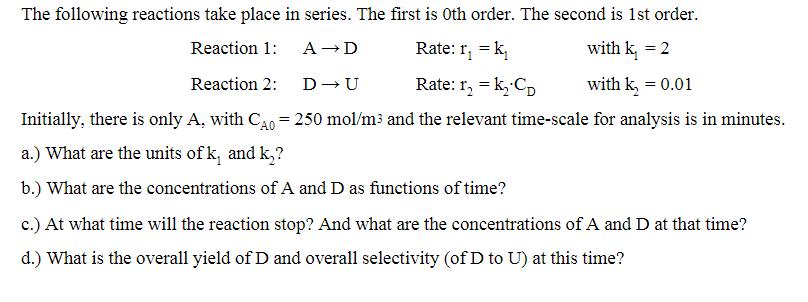
The following reactions take place in series. The first is Oth order. The second is 1st order. Reaction 1: A D Reaction 2: D-U Rate: r = k Rate: 1 = k CD with k = 2 with k = 0.01 Initially, there is only A, with CA0 = 250 mol/m and the relevant time-scale for analysis is in minutes. a.) What are the units of k and k? b.) What are the concentrations of A and D as functions of time? c.) At what time will the reaction stop? And what are the concentrations of A and D at that time? d.) What is the overall yield of D and overall selectivity (of D to U) at this time?
Step by Step Solution
3.27 Rating (156 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Elements Of Chemical Reaction Engineering
Authors: H. Fogler
6th Edition
013548622X, 978-0135486221
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



