Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Let's re-do the parallel reactions example from class (i.e. Example 8.1). Use the same reactions, exception the reaction kinetics for reaction 2 are as
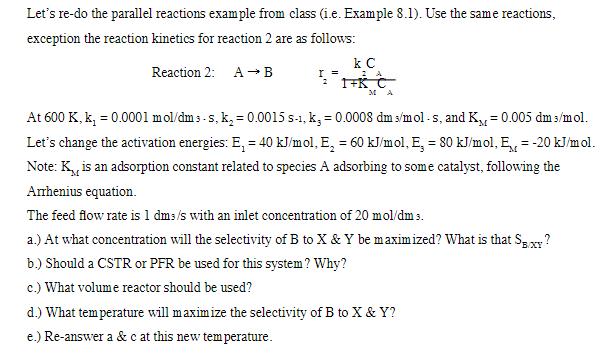
Let's re-do the parallel reactions example from class (i.e. Example 8.1). Use the same reactions, exception the reaction kinetics for reaction 2 are as follows: Reaction 2: AB I = k C 2 MA At 600 K, k = 0.0001 mol/dms-s, k = 0.0015 s-1, k, = 0.0008 dm s/mol-s, and K = 0.005 dm/mol. Let's change the activation energies: E = 40 kJ/mol, E, = 60 kJ/mol, E, = 80 kJ/mol, E = -20 kJ/mol. Note: K,, is an adsorption constant related to species A adsorbing to some catalyst, following the Arrhenius equation. The feed flow rate is 1 dms/s with an inlet concentration of 20 mol/dm 5. a.) At what concentration will the selectivity of B to X & Y be maximized? What is that SBXY? b.) Should a CSTR or PFR be used for this system? Why? c.) What volume reactor should be used? d.) What temperature will maximize the selectivity of B to X & Y? e.) Re-answer a &c at this new temperature.
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Lets redo the parallel reactions example using the new reaction kinetics for reaction 2 Reaction 1 A ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


