Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The following reactors in sequence are used to convert methane to hydrogen: (i) Steam methane reforming: CH4+H2O=CO+3H2; endothermic; 750800C (ii) High temperature water gas shift:
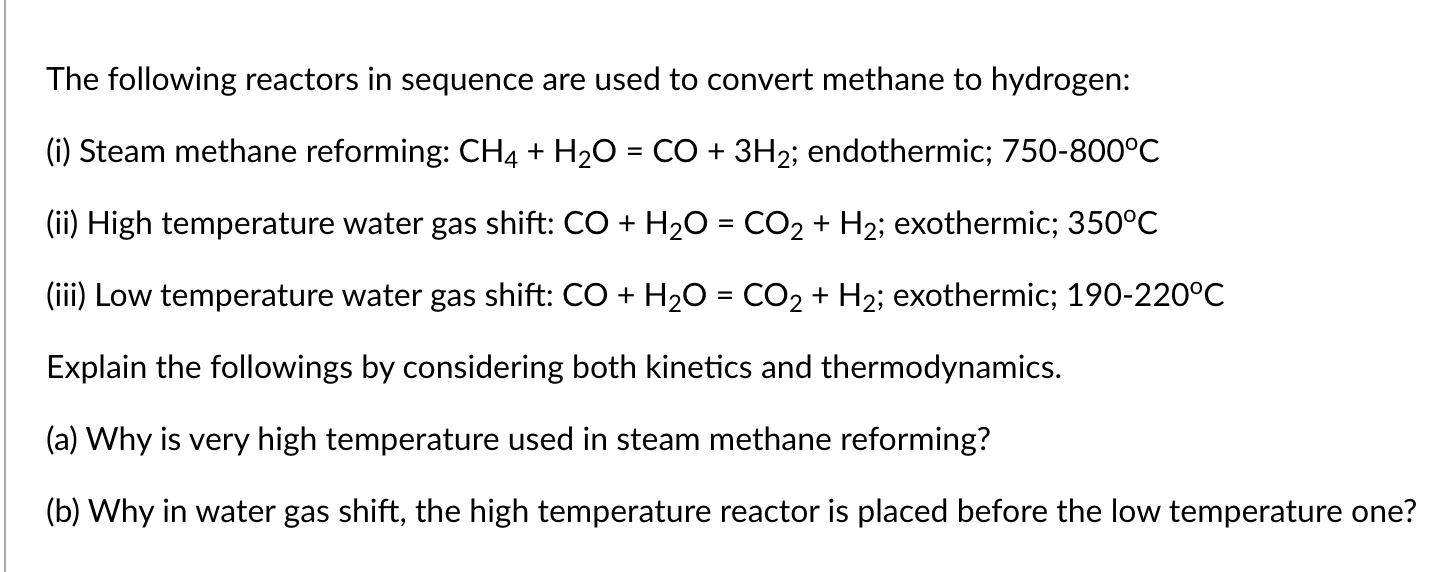 The following reactors in sequence are used to convert methane to hydrogen: (i) Steam methane reforming: CH4+H2O=CO+3H2; endothermic; 750800C (ii) High temperature water gas shift: CO+H2O=CO2+H2; exothermic; 350C (iii) Low temperature water gas shift: CO+H2O=CO2+H2; exothermic; 190-220 C Explain the followings by considering both kinetics and thermodynamics. (a) Why is very high temperature used in steam methane reforming? (b) Why in water gas shift, the high temperature reactor is placed before the low temperature one
The following reactors in sequence are used to convert methane to hydrogen: (i) Steam methane reforming: CH4+H2O=CO+3H2; endothermic; 750800C (ii) High temperature water gas shift: CO+H2O=CO2+H2; exothermic; 350C (iii) Low temperature water gas shift: CO+H2O=CO2+H2; exothermic; 190-220 C Explain the followings by considering both kinetics and thermodynamics. (a) Why is very high temperature used in steam methane reforming? (b) Why in water gas shift, the high temperature reactor is placed before the low temperature one Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


