Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The heat of mixing data for a binary mixture of tetramethyl-1,3-butanediamine (TMBD) and n-heptane at 298.15K were correlated by Dahmani et al. (2002) in the
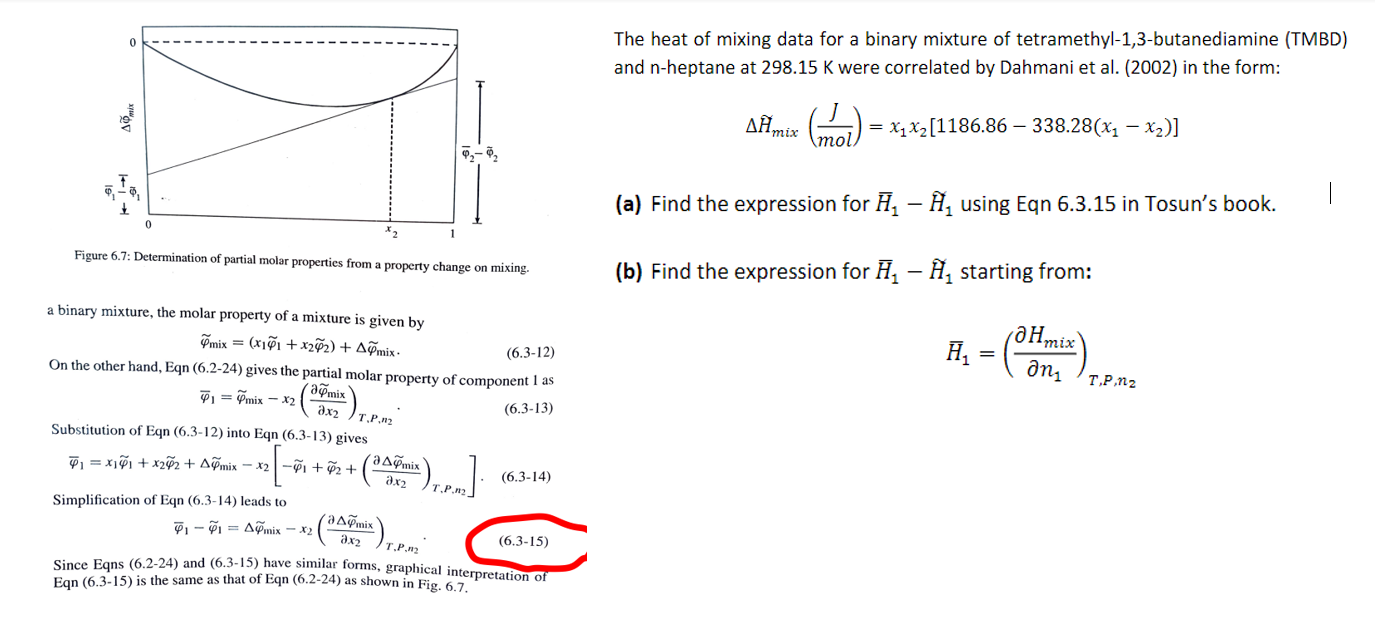 The heat of mixing data for a binary mixture of tetramethyl-1,3-butanediamine (TMBD) and n-heptane at 298.15K were correlated by Dahmani et al. (2002) in the form: Hmix(molJ)=x1x2[1186.86338.28(x1x2)] (a) Find the expression for H1H1 using Eqn 6.3 .15 in Tosun's book. Figure 6.7: Determination of partial molar properties from a property change on mixing. a binary mixture, the molar property of a mixture is given by mix=(x1~1+x22)+mix On the other hand, Eqn (6.2-24) gives the partial molar property of component 1 as 1=mixx2(x2~mix)T,P,n2 Substitution of Eqn (6.3-12) into Eqn (6.3-13) gives SubstitutionofEqn(6.3-12)intoEqn(6.3-13)gives1=x11+x22+mixx2[1+2+(x2mix)T,P,n2]. Simplification of Eqn (6.3-14) leads to 11=mixx2(x2mix)T,P,n2 (6.315) Since Eqns (6.2-24) and (6.3-15) have similar forms, graphical interpretation of (b) Find the expression for H1H1 starting from: H1=(n1Hmix)T,P,n2
The heat of mixing data for a binary mixture of tetramethyl-1,3-butanediamine (TMBD) and n-heptane at 298.15K were correlated by Dahmani et al. (2002) in the form: Hmix(molJ)=x1x2[1186.86338.28(x1x2)] (a) Find the expression for H1H1 using Eqn 6.3 .15 in Tosun's book. Figure 6.7: Determination of partial molar properties from a property change on mixing. a binary mixture, the molar property of a mixture is given by mix=(x1~1+x22)+mix On the other hand, Eqn (6.2-24) gives the partial molar property of component 1 as 1=mixx2(x2~mix)T,P,n2 Substitution of Eqn (6.3-12) into Eqn (6.3-13) gives SubstitutionofEqn(6.3-12)intoEqn(6.3-13)gives1=x11+x22+mixx2[1+2+(x2mix)T,P,n2]. Simplification of Eqn (6.3-14) leads to 11=mixx2(x2mix)T,P,n2 (6.315) Since Eqns (6.2-24) and (6.3-15) have similar forms, graphical interpretation of (b) Find the expression for H1H1 starting from: H1=(n1Hmix)T,P,n2 Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


