Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The hydrolysis of y-valerolactone to y-hydroxyvaleric acid is to be performed in the aqueous phase. + HO +B HO. CH3 OH A The reaction
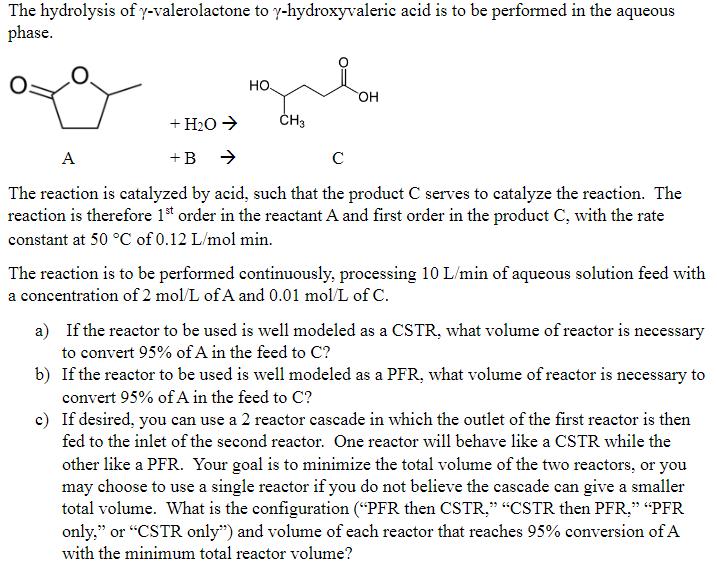
The hydrolysis of y-valerolactone to y-hydroxyvaleric acid is to be performed in the aqueous phase. + HO +B HO. CH3 OH A The reaction is catalyzed by acid, such that the product C serves to catalyze the reaction. The reaction is therefore 1st order in the reactant A and first order in the product C, with the rate constant at 50 C of 0.12 L/mol min. The reaction is to be performed continuously, processing 10 L/min of aqueous solution feed with a concentration of 2 mol/L of A and 0.01 mol/L of C. a) If the reactor to be used is well modeled as a CSTR, what volume of reactor is necessary to convert 95% of A in the feed to C? b) If the reactor to be used is well modeled as a PFR, what volume of reactor is necessary to convert 95% of A in the feed to C? c) If desired, you can use a 2 reactor cascade in which the outlet of the first reactor is then fed to the inlet of the second reactor. One reactor will behave like a CSTR while the other like a PFR. Your goal is to minimize the total volume of the two reactors, or you may choose to use a single reactor if you do not believe the cascade can give a smaller total volume. What is the configuration ("PFR then CSTR." "CSTR then PFR," "PFR only," or "CSTR only") and volume of each reactor that reaches 95% conversion of A with the minimum total reactor volume?
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


