Question: How would you modify Table 6-2 for a. A constant-volume gas-phase reaction, and b. A variable-volume gas-phase reaction? Table 6-2 1. Mole balances: BR dNAdt=rAV
How would you modify Table 6-2 for
a. A constant-volume gas-phase reaction, and
b. A variable-volume gas-phase reaction?
Table 6-2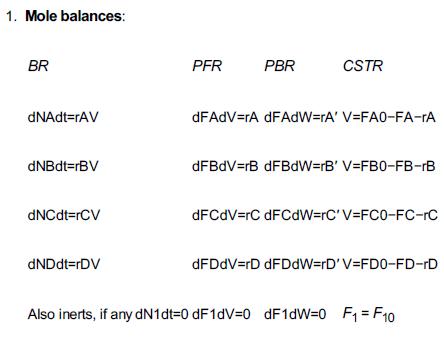

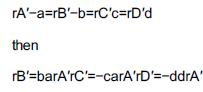

1. Mole balances: BR dNAdt=rAV dNBdt=rBV dNCdt=rCV dNDdt=rDV PFR PBR CSTR dFAdV=rA dFAdW=rA' V=FAO-FA-rA dFBdV=rB dFBdW=rB' V=FB0-FB-rB dFCdV=rC dFCdW=rC'V=FC0-FC-rC dFDdV=rD dFDdW=rD' V=FD0-FD-rD Also inerts, if any dN1dt=0 dF1dV=0 dF1dW=0 F=F10
Step by Step Solution
3.33 Rating (153 Votes )
There are 3 Steps involved in it
The mole balances and rates remain the same Only th... View full answer

Get step-by-step solutions from verified subject matter experts


