Question
What is the predominant intermolecular force in CH3NHCH3? dipole-dipole forces O Hydrogen bonding O London dispersion forces Olon-dipole ineractions O lonic bonding
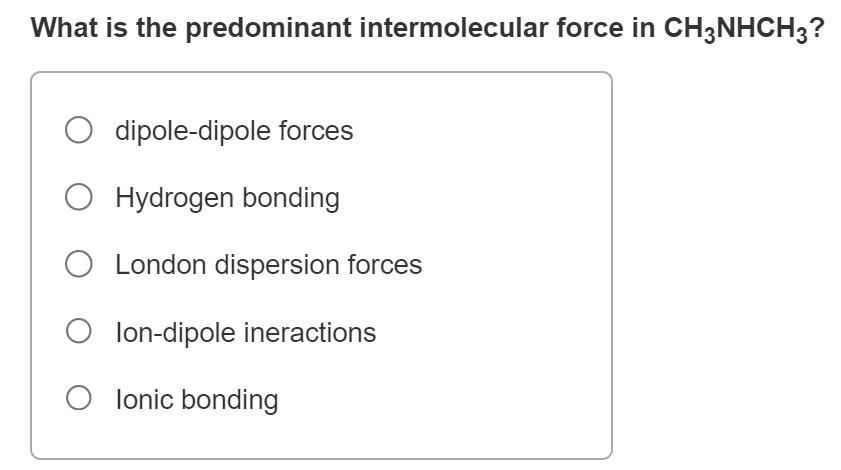
What is the predominant intermolecular force in CH3NHCH3? dipole-dipole forces O Hydrogen bonding O London dispersion forces Olon-dipole ineractions O lonic bonding
Step by Step Solution
3.38 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1
London dispersion forces London dispersion forces are a type of ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Financial Reporting and Analysis
Authors: Flawrence Revsine, Daniel Collins, Bruce, Mittelstaedt, Leon
6th edition
9780077632182, 78025672, 77632184, 978-0078025679
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



