Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The industrial production of lime (CaO) from calcium carbonate is accomplished via the following reaction: CaCO3(s)CaO(s)+CO2(g) Given the following data: what can be said about
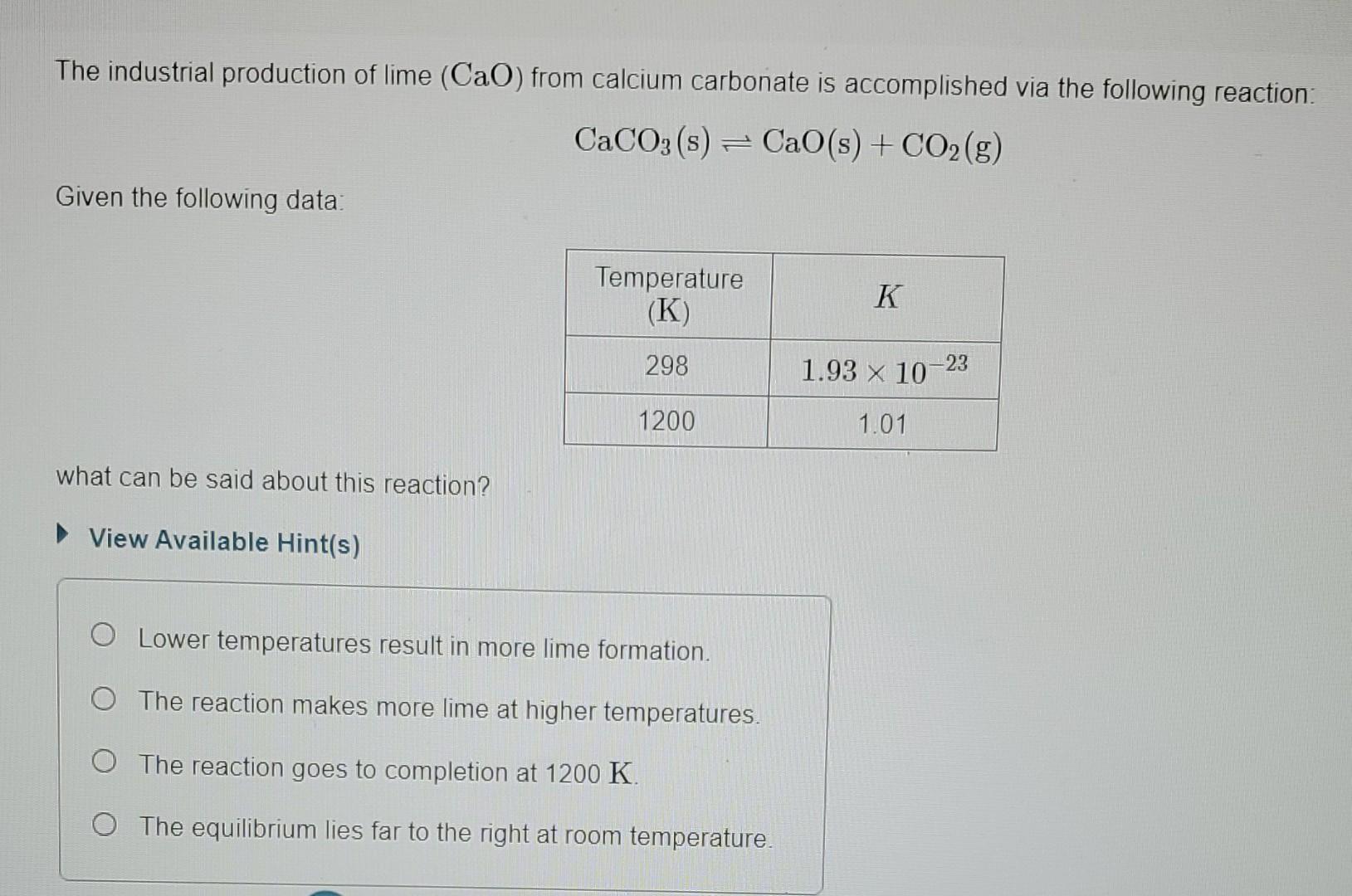
The industrial production of lime (CaO) from calcium carbonate is accomplished via the following reaction: CaCO3(s)CaO(s)+CO2(g) Given the following data: what can be said about this reaction? View Available Hint(s) Lower temperatures result in more lime formation. The reaction makes more lime at higher temperatures. The reaction goes to completion at 1200K. The equilibrium lies far to the right at room temperature
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


