Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The irreversible gas-phase dimeriztion A1/2A2 is carried out at 8.2 atm in a stirred contained-solids reactor to which only pure A is fed. There is
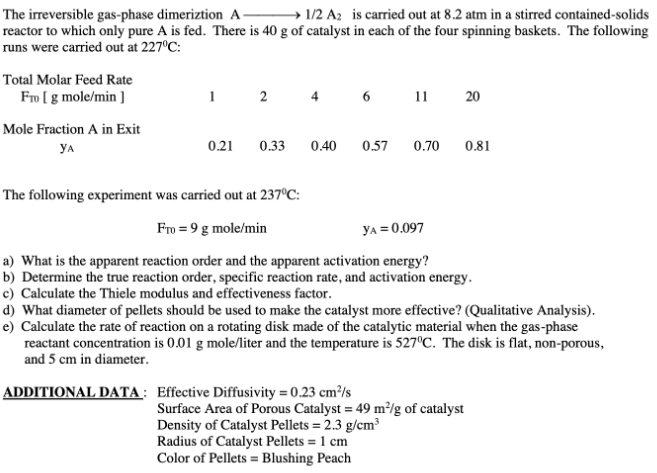 The irreversible gas-phase dimeriztion A1/2A2 is carried out at 8.2 atm in a stirred contained-solids reactor to which only pure A is fed. There is 40g of catalyst in each of the four spinning baskets. The following runs were carried out at 227C : The following experiment was carried out at 237C : FT0=9gmole/minyA=0.097 a) What is the apparent reaction order and the apparent activation energy? b) Determine the true reaction order, specific reaction rate, and activation energy. c) Calculate the Thiele modulus and effectiveness factor. d) What diameter of pellets should be used to make the catalyst more effective? (Qualitative Analysis). e) Calculate the rate of reaction on a rotating disk made of the catalytic material when the gas-phase reactant concentration is 0.01g mole/liter and the temperature is 527C. The disk is flat, non-porous, and 5cm in diameter. ADDITIONAL DATA : Effective Diffusivity =0.23cm2/s Surface Area of Porous Catalyst =49m2/g of catalyst
The irreversible gas-phase dimeriztion A1/2A2 is carried out at 8.2 atm in a stirred contained-solids reactor to which only pure A is fed. There is 40g of catalyst in each of the four spinning baskets. The following runs were carried out at 227C : The following experiment was carried out at 237C : FT0=9gmole/minyA=0.097 a) What is the apparent reaction order and the apparent activation energy? b) Determine the true reaction order, specific reaction rate, and activation energy. c) Calculate the Thiele modulus and effectiveness factor. d) What diameter of pellets should be used to make the catalyst more effective? (Qualitative Analysis). e) Calculate the rate of reaction on a rotating disk made of the catalytic material when the gas-phase reactant concentration is 0.01g mole/liter and the temperature is 527C. The disk is flat, non-porous, and 5cm in diameter. ADDITIONAL DATA : Effective Diffusivity =0.23cm2/s Surface Area of Porous Catalyst =49m2/g of catalyst Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


