THE LAB IS INCLUDED STRICTLY FOR DETAILS. I AM ONLY LOOKING FOR THE 5 DIAGRAMS...
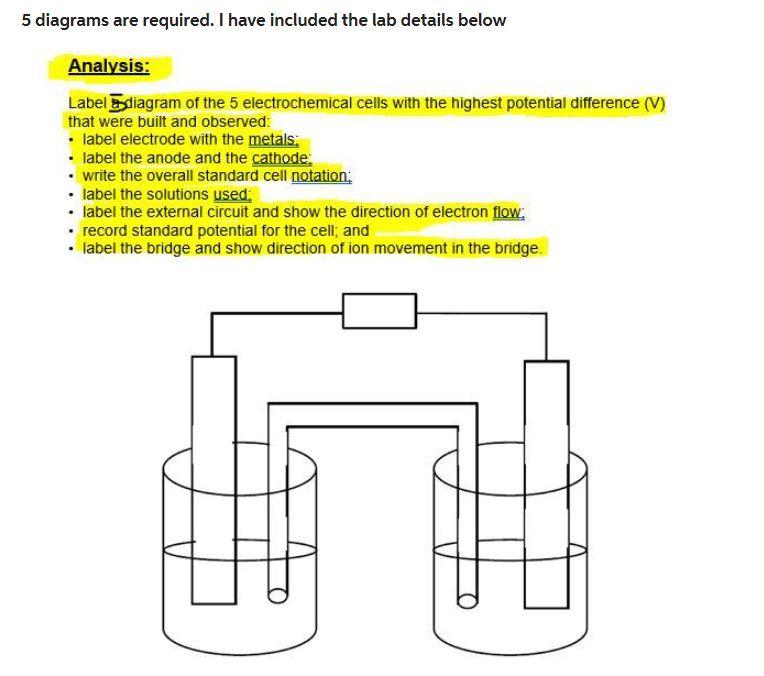

5 diagrams are required. I have included the lab details below Analysis: Label Sydiagram of the 5 electrochemical cells with the highest potential difference (V) that were built and observed: - label electrode with the metals: - label the anode and the cathode: - write the overall standard cell notation: - label the solutions used. - label the external circuit and show the direction of electron flow: - record standard potential for the cell; and - label the bridge and show direction of ion movement in the bridge. Introduction: Please include the following information in your introduction: Describe a galvanic cell and how it functions. Make sure to include all the relevant parts. How is the anode and cathode determined using redox table. How is the electrical potential determined for a cell? Explain redox reactions and half reactions. Purpose: The purpose of this lab is to build a galvanic cells, draw a labelled diagram of the cells showing the oxidation-reduction reactions, the direction of electron flow, the electrode polarity, the cell potential, and the direction of ion movement. Procedure: 1. You can select the temperature using the slider. Set the temperature at 25C 2. You can select the anode from 'Select Electrode' dropdown list. 3. You can select the concentration of the electrolyte in the anodic part using the slider. Set the concentration at 0.1M. 4. Now, select the cathode from 'Select Electrode' dropdown list. 5. To select the concentration of the electrolyte in the cathodic part use its slider. Set the concentration at 0.1M. 6. You can see the value of emf of each cell on the voltmeter. 7. You can also see the chemical reactions and the equation to calculate the EMF of the cell on the left side menu. 8. To redo the experiment anytime, click on the 'Reset' button. Observations: 5 diagrams are required. I have included the lab details below Analysis: Label Sydiagram of the 5 electrochemical cells with the highest potential difference (V) that were built and observed: - label electrode with the metals: - label the anode and the cathode: - write the overall standard cell notation: - label the solutions used. - label the external circuit and show the direction of electron flow: - record standard potential for the cell; and - label the bridge and show direction of ion movement in the bridge. Introduction: Please include the following information in your introduction: Describe a galvanic cell and how it functions. Make sure to include all the relevant parts. How is the anode and cathode determined using redox table. How is the electrical potential determined for a cell? Explain redox reactions and half reactions. Purpose: The purpose of this lab is to build a galvanic cells, draw a labelled diagram of the cells showing the oxidation-reduction reactions, the direction of electron flow, the electrode polarity, the cell potential, and the direction of ion movement. Procedure: 1. You can select the temperature using the slider. Set the temperature at 25C 2. You can select the anode from 'Select Electrode' dropdown list. 3. You can select the concentration of the electrolyte in the anodic part using the slider. Set the concentration at 0.1M. 4. Now, select the cathode from 'Select Electrode' dropdown list. 5. To select the concentration of the electrolyte in the cathodic part use its slider. Set the concentration at 0.1M. 6. You can see the value of emf of each cell on the voltmeter. 7. You can also see the chemical reactions and the equation to calculate the EMF of the cell on the left side menu. 8. To redo the experiment anytime, click on the 'Reset' button. Observations








