Question
The Lewis structures of four compounds are given. -0 PC13 CO SO CHCl2 H + Which of these molecules are polar? :C: -ci: For
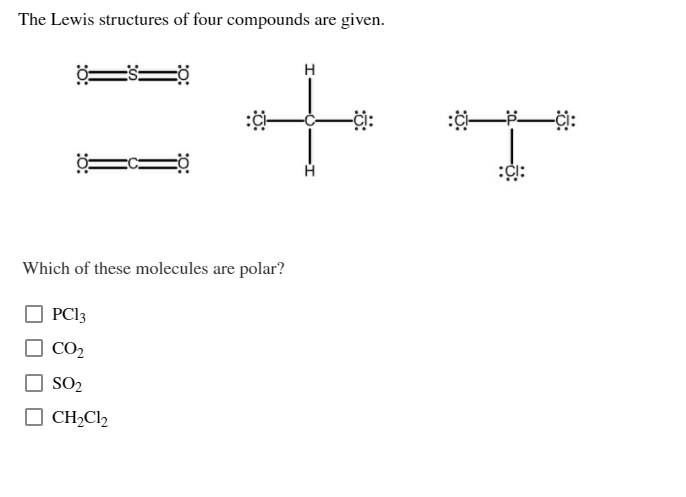
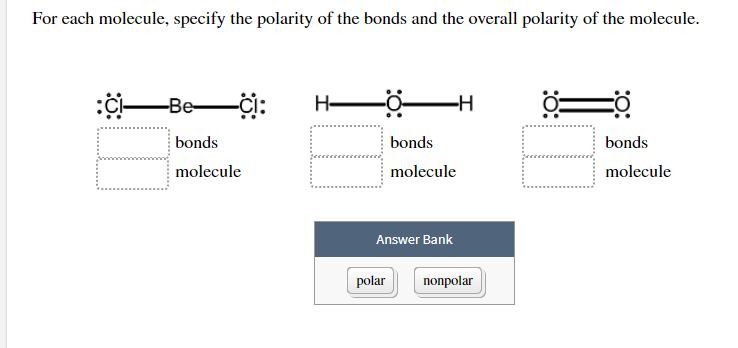
The Lewis structures of four compounds are given. -0 PC13 CO SO CHCl2 H + Which of these molecules are polar? :C: -ci: For each molecule, specify the polarity of the bonds and the overall polarity of the molecule. :CI Be bonds molecule H- -H polar bonds molecule Answer Bank nonpolar 0= bonds molecule
Step by Step Solution
3.52 Rating (166 Votes )
There are 3 Steps involved in it
Step: 1
1 Anser PCl3 Explanation PCl3 molecule is polar nature Because its asymmetric structure and differen...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Organic Chemistry structure and function
Authors: K. Peter C. Vollhardt, Neil E. Schore
6th edition
142920494X, 978-1429204941
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



