Answered step by step
Verified Expert Solution
Question
1 Approved Answer
The liquid phase reversible reaction R T is carried out in an ideal mixed flow reactor. The concentrations of R and T that enter
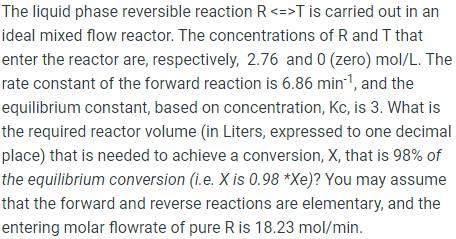
The liquid phase reversible reaction R T is carried out in an ideal mixed flow reactor. The concentrations of R and T that enter the reactor are, respectively, 2.76 and 0 (zero) mol/L. The rate constant of the forward reaction is 6.86 min-, and the equilibrium constant, based on concentration, Kc, is 3. What is the required reactor volume (in Liters, expressed to one decimal place) that is needed to achieve a conversion, X, that is 98% of the equilibrium conversion (i.e. X is 0.98 *Xe)? You may assume that the forward and reverse reactions are elementary, and the entering molar flowrate of pure R is 18.23 mol/min.
Step by Step Solution
★★★★★
3.42 Rating (146 Votes )
There are 3 Steps involved in it
Step: 1
SOLUTION To achieve a conversion of 98 of the equilibrium conversion we need to reach a point where ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


