Answered step by step
Verified Expert Solution
Question
1 Approved Answer
19. The metal tin undergoes a transition from a gray phase to a white phase at 286 K and atmospheric pressure. Given that the
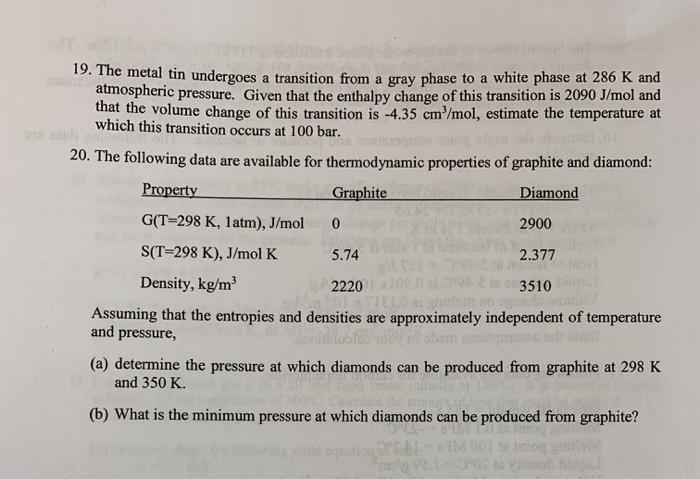
19. The metal tin undergoes a transition from a gray phase to a white phase at 286 K and atmospheric pressure. Given that the enthalpy change of this transition is 2090 J/mol and that the volume change of this transition is -4.35 cm/mol, estimate the temperature at which this transition occurs at 100 bar. 20. The following data are available for thermodynamic properties of graphite and diamond: Property Graphite Diamond G(T=298 K, latm), J/mol 2900 S(T=298 K), J/mol K 5.74 2.377 Density, kg/m 2220 3510 Assuming that the entropies and densities are approximately independent of temperature and pressure, (a) determine the pressure at which diamonds can be produced from graphite at 298 K and 350 K. (b) What is the minimum pressure at which diamonds can be produced from graphite?
Step by Step Solution
★★★★★
3.35 Rating (161 Votes )
There are 3 Steps involved in it
Step: 1
From Eq 574 V bar 10Pa 99bar 435 286K mole AT 5...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


