Answered step by step
Verified Expert Solution
Question
1 Approved Answer
the pictures are in order and i want to know what is the last 5 rows please help me explain it carefull thanks alot this



the pictures are in order and i want to know what is the last 5 rows please help me explain it carefull thanks alot
this is the actual given table 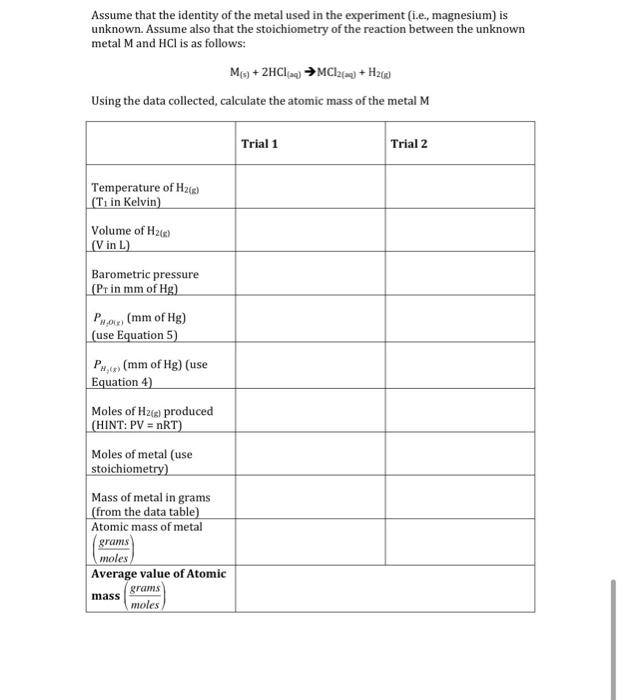
Data Table moss & mg & Torial 1 0.04629 Toial 2 0.04489 Ved High O. 60441 L NAS. 0.04555L Temperate of hopik 295.15k 295.15k 749 749 Barometric pressure mom of Hg 23mm height difference between endiameter and graduated cylinder G calculations mas & macg 0.04629 o & quog (24.305glms! Alric mas of Maland) 24 305 g/mol 1.9 onio und incles of Mg 1.82 10-3 md Moles of H cg) 1.96 10 mol 181210-3 mo! 295 isk tolure & g) 0.04555aL i dilemperatie of Hug)Q) 295.5K C12 1 8001412 (hind Barometric Pressure (P remom of Hg) Prog) (mm & Hg) 749 749 19.83 19.83 229.17 729.17 0 04361 Phyco (Some P) (mm of H) volume of Heg at STP (V in L. Moda Volume. Ata at STP CL/mol) 0.04364 22.9 Limol 24.1 L/mol Percent error' 3.6% 10.1% Trial 2 295.15k 0.09555L 749mm Abanic mass of metal We Trial I Temperature of the 295.15k (Tiinkenn) volume of thes) 10.04414 (Vina) Borometric pressure 74aar CPi nmn of Ag) Phacg) (mm A Hg) Przeg (mm sty) modes of legopodal moles of metal mm 19.83hm 19.83 729. 17m 729 17mm mass of metaling Atomic mas Anetal g/mol) Aerage volume & Atomic mass (almol) Assume that the identity of the metal used in the experiment (i.e., magnesium) is unknown. Assume also that the stoichiometry of the reaction between the unknown metal M and HCl is as follows: Mg + 2HC124) MC12(22) + H20 Using the data collected, calculate the atomic mass of the metal M Trial 1 Trial 2 Temperature of H2) (T in Kelvin) Volume of H26 (V in L) Barometric pressure (Prin mm of Hg) Pou (mm of Hg) (use Equation 5) PH (mm of Hg) (use Equation 4) Moles of H2(e) produced (HINT: PV = nRT) Moles of metal (use stoichiometry) Mass of metal in grams (from the data table) Atomic mass of metal grams moles Average value of Atomic grams mass moles 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


