Question
The rate of reaction between magnesium and excess dilute hydrochloric acid was followed by measuring the mass of magnesium present at regular time intervals, two
The rate of reaction between magnesium and excess dilute hydrochloric acid was followed by measuring the mass of magnesium present at regular time intervals, two experiments were performed. Both experiments used 0.1 g of magnesium ribbon. The acid in experiment 1. was less concentrated than experiments 2,
Which graph shows the results of experiments?
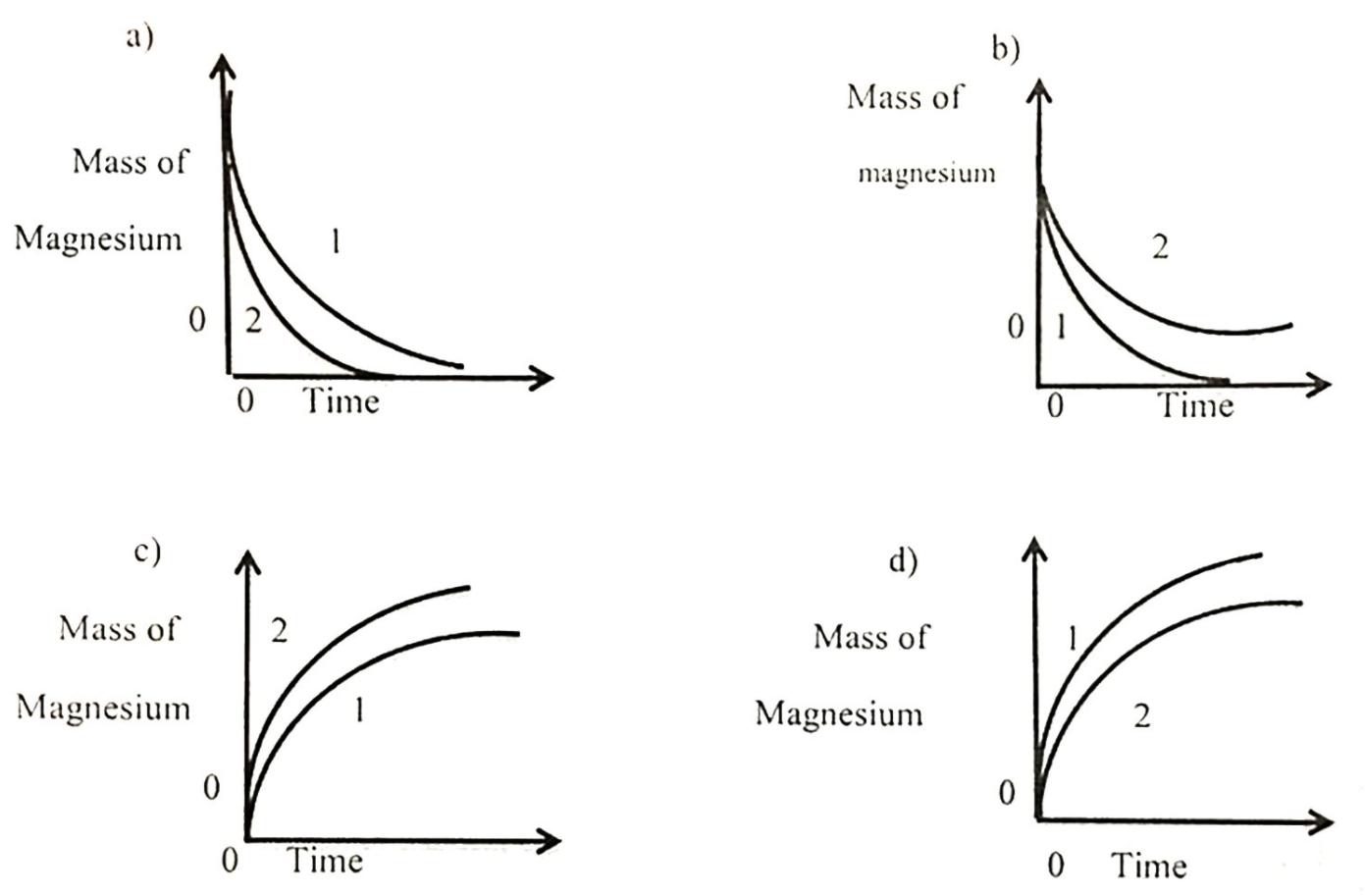
a) b) Mass of Mass of magnesium Magnesium 2 0|1 Time Time c) d) Mass of 2 Mass of Magnesium Magnesium 2 Time Time
Step by Step Solution
3.29 Rating (149 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Organic Chemistry
Authors: Paula Yurkanis Bruice
4th edition
131407481, 978-0131407480
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



