Question
The rate of self-discharge is a critical design parameter for primary batteries. 1) Assume that a primary battery is designed to last for 5 years
The rate of self-discharge is a critical design parameter for primary batteries.
1) Assume that a primary battery is designed to last for 5 years at a constant average discharge rate. At what C-rate does this battery operate?
2) For this same cell, what is the equivalent C-rate for the self-discharge process if the current efficiency is to be kept above 90%? Assume that the self-discharge reaction operates as a chemical short in parallel with the main electrochemical reaction.
3) Because of the extremely long lives of some batteries, microcalorimetry is used to measure the rate of self-discharge. Data for a Li/I2 cell are shown on the right. Estimate the current efficiency of the discharge. The equilibrium potential is 2.80V, the cell resistance is 650, and the entropic contribution is 0.0092 J.C-1 at the cell temperature. Assume a nominal operating current of 70 A.
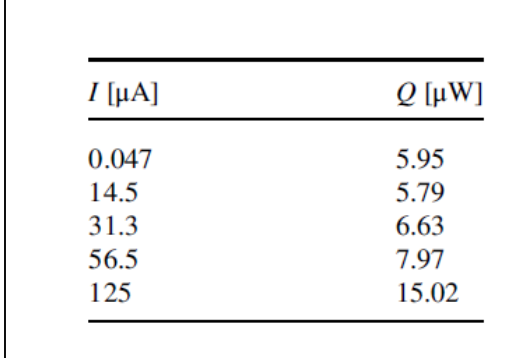 \begin{tabular}{ll} \hlineI[A] & Q[W] \\ \hline 0.047 & 5.95 \\ 14.5 & 5.79 \\ 31.3 & 6.63 \\ 56.5 & 7.97 \\ 125 & 15.02 \\ \hline \end{tabular} \begin{tabular}{ll} \hlineI[A] & Q[W] \\ \hline 0.047 & 5.95 \\ 14.5 & 5.79 \\ 31.3 & 6.63 \\ 56.5 & 7.97 \\ 125 & 15.02 \\ \hline \end{tabular}
\begin{tabular}{ll} \hlineI[A] & Q[W] \\ \hline 0.047 & 5.95 \\ 14.5 & 5.79 \\ 31.3 & 6.63 \\ 56.5 & 7.97 \\ 125 & 15.02 \\ \hline \end{tabular} \begin{tabular}{ll} \hlineI[A] & Q[W] \\ \hline 0.047 & 5.95 \\ 14.5 & 5.79 \\ 31.3 & 6.63 \\ 56.5 & 7.97 \\ 125 & 15.02 \\ \hline \end{tabular} Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


