Answered step by step
Verified Expert Solution
Question
1 Approved Answer
the temperature reagent is 26.0c please use this information to complete the table. please help me out by complete solution for all this question. (2pts)
the temperature reagent is 26.0c

please use this information to complete the table. please help me out by complete solution for all this question.
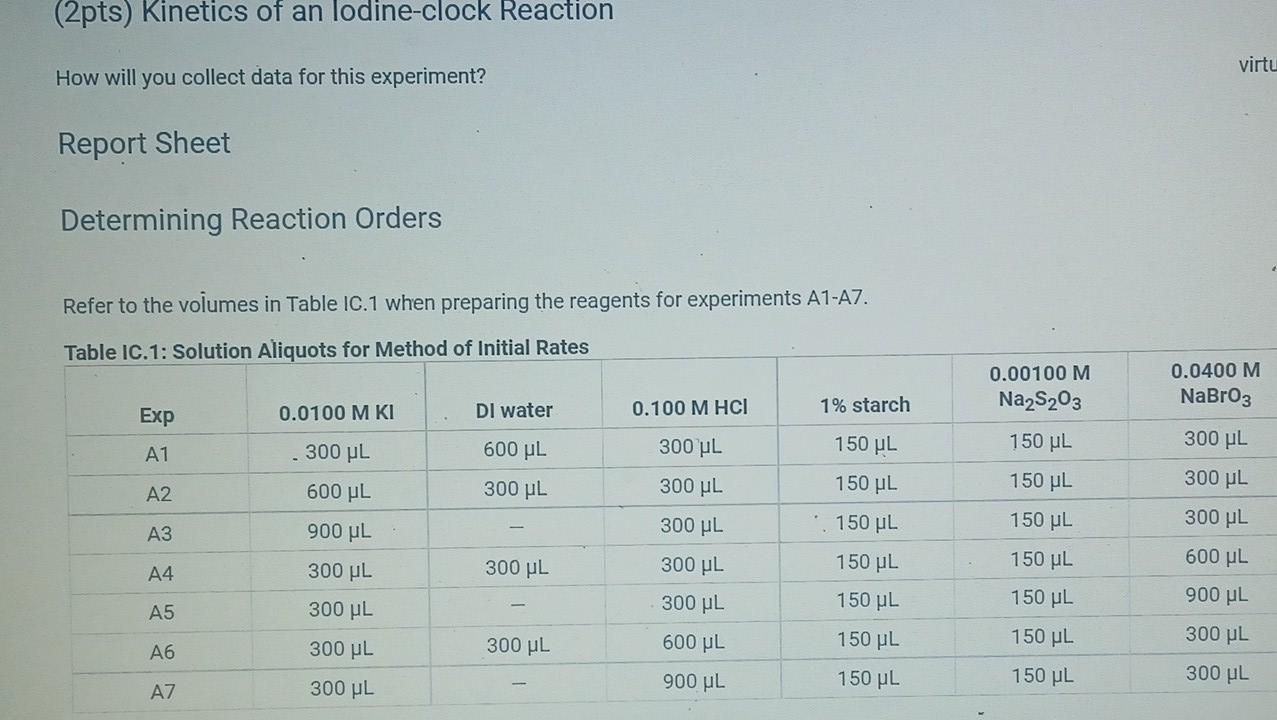
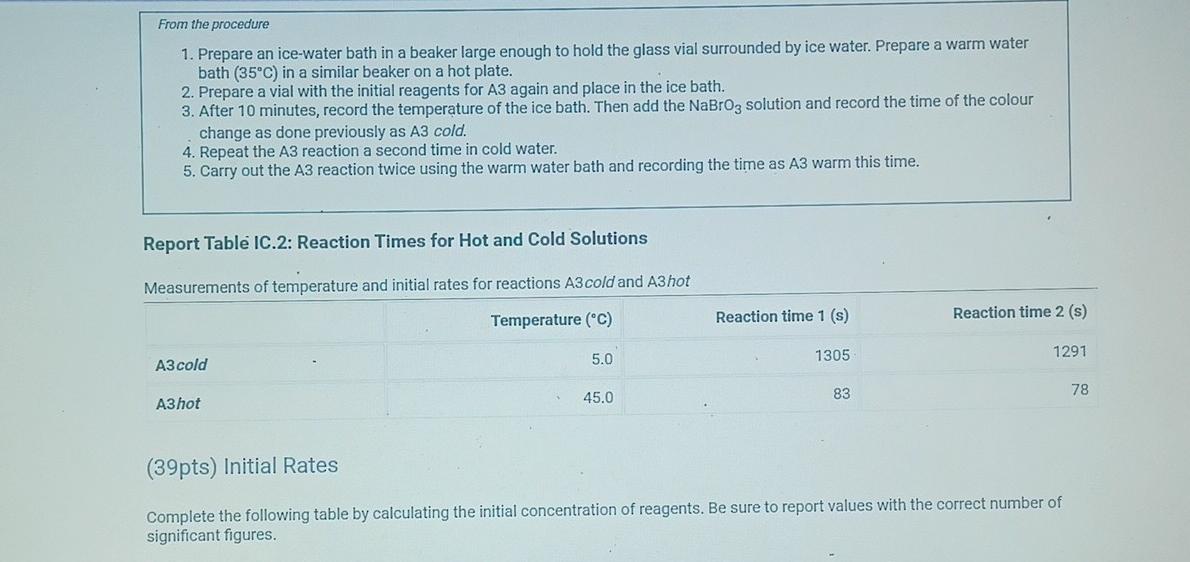
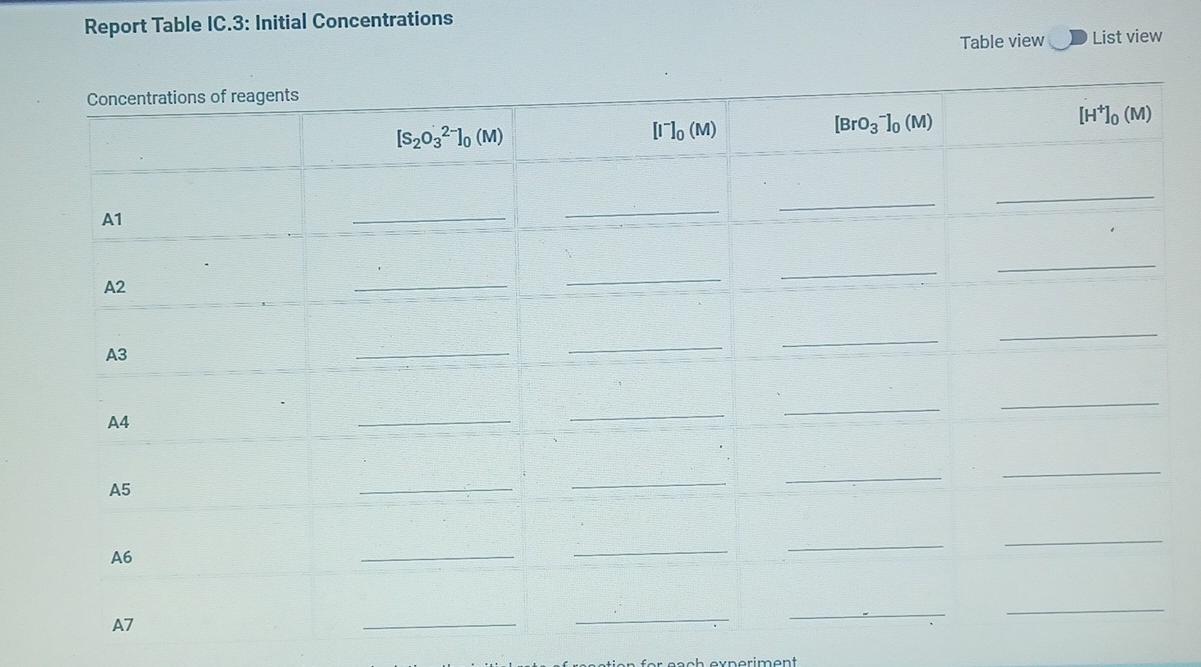
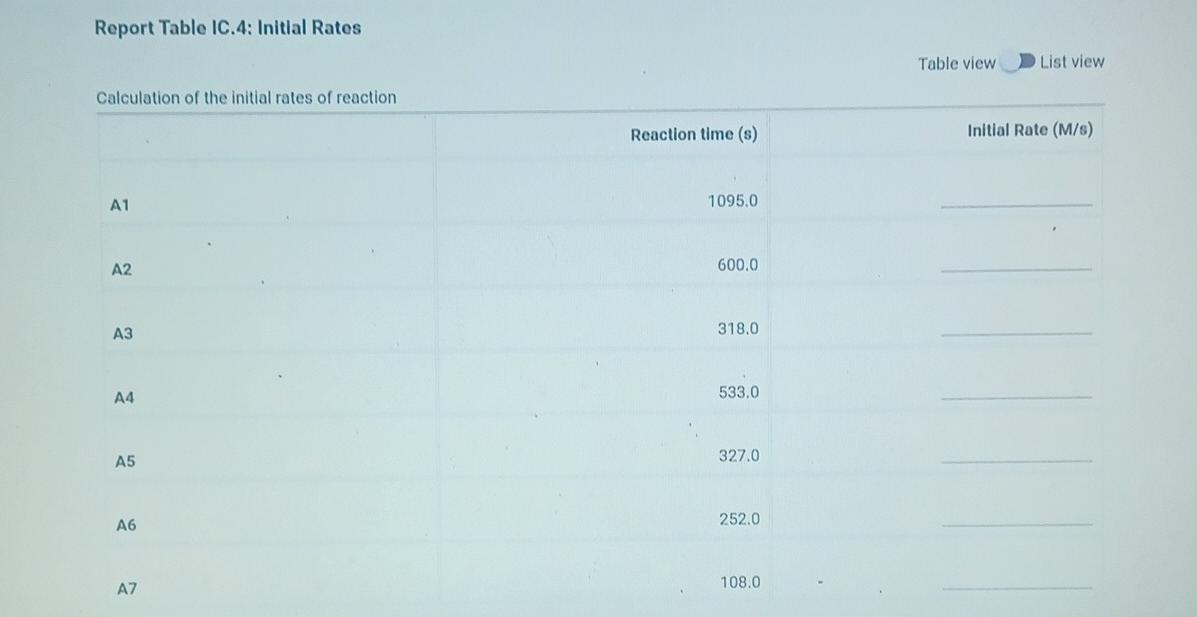
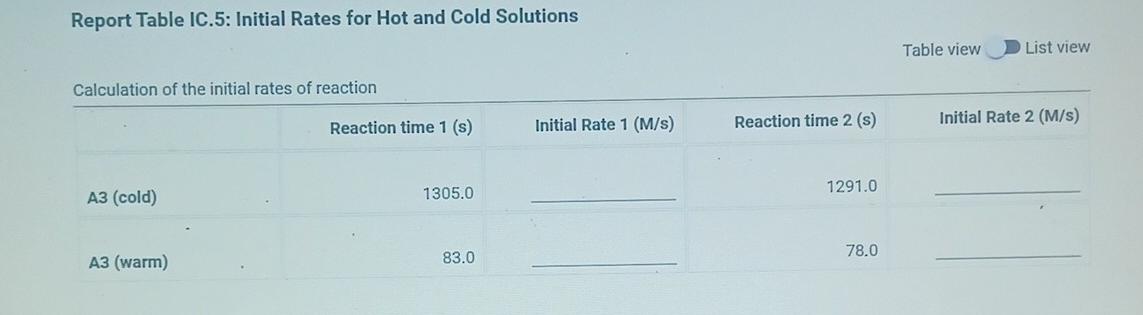
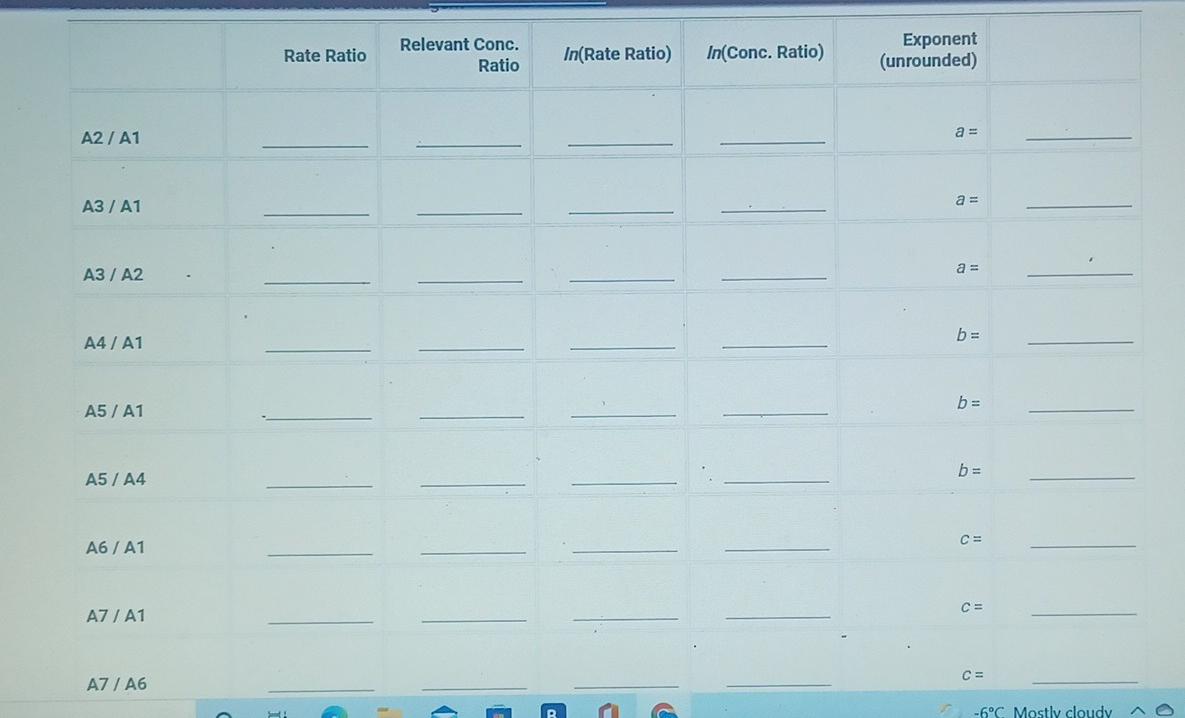
(2pts) Kinetics of an lodine-clock Reaction virtu How will you collect data for this experiment? Report Sheet Determining Reaction Orders Refer to the volumes in Table IC.1 when preparing the reagents for experiments A1-A7. Table IC.1: Solution Aliquots for Method of Initial Rates 0.00100 M Na2S203 0.0400 M NaBrO3 Exp 0.0100 MKI Dl water 0.100 M HCl 1% starch 300 PL 600 PL A1 300 HL 150 UL 150 UL 300 PL A2 150 L 300 ML 600 PL 300 PL 300 UL 150 PL 150 UL 150 ML 300 UL A3 300 PL 900 PL A4 150 L 600 PL 300 PL 300 PL 150 PL 300 PL 300 PL 150 UL 150 UL A5 300 ML 900 PL 300 PL 300 PL A6 300 ML 600 PL 150 PL 150 PL 900 ML 150 ML 150 PL 300 UL 300 UL AZ It a stop-watch that reported time to the nundretns of a second read 01:42:01, the time would be repor Report Table IC.1: Reaction Times Reaction times for experiments A1-A7 Reaction time (s) A1 1095 A2 600 318 A4 533 A5 327 A6 252 AZ 108 From the procedure 1. Prepare an ice-water bath in a beaker large enough to hold the glass vial surrounded by ice water. Prepare a warm water bath (35C) in a similar beaker on a hot plate. 2. Prepare a vial with the initial reagents for A3 again and place in the ice bath. 3. After 10 minutes, record the temperature of the ice bath. Then add the NaBrog solution and record the time of the colour change as done previously as A3 cold. 4. Repeat the A3 reaction a second time in cold water. 5. Carry out the A3 reaction twice using the warm water bath and recording the time as A3 warm this time. Report Table IC.2: Reaction Times for Hot and Cold Solutions Measurements of temperature and initial rates for reactions A3 cold and A3 hot Temperature (C) Reaction time 1 (s) Reaction time 2 (s) 5.0 1305 1291 A3 cold 45.0 83 78 hot (39pts) Initial Rates Complete the following table by calculating the initial concentration of reagents. Be sure to report values with the correct number of significant figures. Report Table IC.3: Initial Concentrations Table view List view Concentrations of reagents [Broz 10 (M) [H*1. (M) [S2032-1, (M) [1 l. (M) A1 A2 A4 A5 A6 AZ ion for each eyneriment Report Table IC.4: Initial Rates Table view List view Calculation of the initial rates of reaction Reaction time (s) Initial Rate (M/s) A1 1095.0 A2 600.0 318.0 A4 533.0 A5 327.0 A6 252.0 AZ 108.0 Report Table IC.5: Initial Rates for Hot and Cold Solutions Table view List view Calculation of the initial rates of reaction Reaction time 1 Initial Rate 1 (M/s) Reaction time 2 (s) Initial Rate 2 (M/s) 1305.0 1291.0 A3 (cold) 78.0 A3 (warm) 83.0 Rate Ratio Relevant Conc. Ratio In(Rate Ratio) In(Conc. Ratio) Exponent (unrounded) a = A2 / A1 A3 / A1 a = a = A3 / A2 b= A4 / A1 b= A5 / A1 b= A5 / A4 CE A6 / A1 C = A7 / A1 CE A7 / A6 - B -6C Mostly cloudyStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


