Answered step by step
Verified Expert Solution
Question
1 Approved Answer
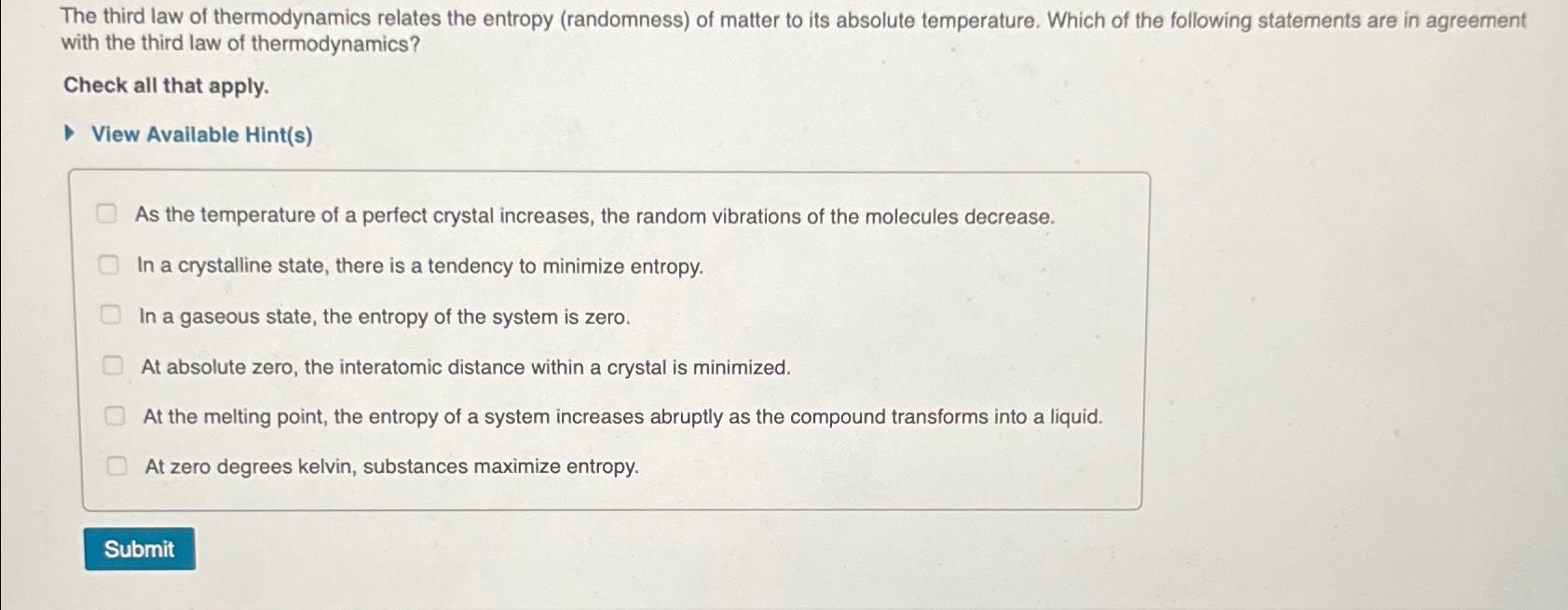
The third law of thermodynamics relates the entropy ( randomness ) of matter to its absolute temperature. Which of the following statements are in agreement
The third law of thermodynamics relates the entropy randomness of matter to its absolute temperature. Which of the following statements are in agreement with the third law of thermodynamics?
Check all that apply.
View Avallable Hints
As the temperature of a perfect crystal increases, the random vibrations of the molecules decrease.
In a crystalline state, there is a tendency to minimize entropy.
In a gaseous state, the entropy of the system is zero.
At absolute zero, the interatomic distance within a crystal is minimized.
At the melting point, the entropy of a system increases abruptly as the compound transforms into a liquid.
At zero degrees kelvin, substances maximize entropy.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


