Answered step by step
Verified Expert Solution
Question
1 Approved Answer
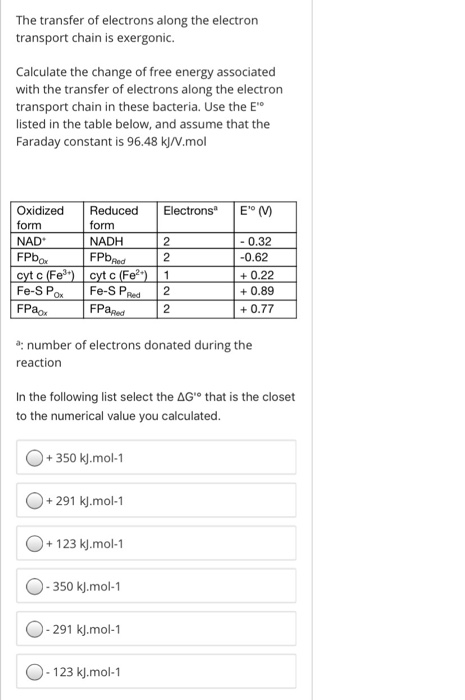
The transfer of electrons along the electron transport chain is exergonic. Calculate the change of free energy associated with the transfer of electrons along the
The transfer of electrons along the electron transport chain is exergonic.
Calculate the change of free energy associated with the transfer of electrons along the electron transport chain in these bacteria. Use the Edeg listed in the table below, and assume that the Faraday constant is kJVmol
Oxidized form
Reduced form
Electrons a
E o V
NAD
NADH
FPb Ox
FPb Red
cyt c Fe
cyt c Fe
FeS P Ox
FeS P Red
FPa Ox
FPa Red
a: number of electrons donated during the reaction
In the following list select the Delta Gdeg that is the closet to the numerical value you calculated.
kJmol
kJmol
kJmol
kJmol
kJmol
kJmol
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


