there is one step missing in physostigmime synthisis. I think is the sodium phenoxide react with N-Succinimidyl-N methylcarbamate. i would like the missing step to be drawn please.
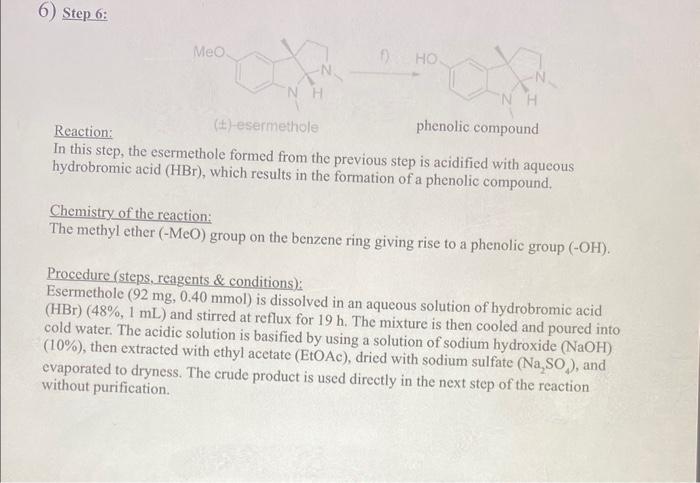
6) Step 6: Reaction: (t)-esermethole phenolic compound In this step, the esermethole formed from the previous step is acidified with aqueous hydrobromic acid (HBr), which results in the formation of a phenolic compound. Chemistry of the reaction: The methyl ether (MeO) group on the benzene ring giving rise to a phenolic group (OH). Procedure (steps, reagents \& conditions): Esermethole (92mg,0.40mmol) is dissolved in an aqueous solution of hydrobromic acid ( HBr)(48%,1mL) and stirred at reflux for 19h. The mixture is then cooled and poured into cold water. The acidic solution is basified by using a solution of sodium hydroxide (NaOH) (10%), then extracted with ethyl acetate (EtOAc), dried with sodium sulfate (Na2SO4), and evaporated to dryness. The crude product is used directly in the next step of the reaction without purification. 7) Step 7: phenolic compound Reaction: In the final step of physostigmine synthesis, the mixture of the phenolic compound from the (yield 44\%) previous reaction (also containing sodium hydride (NaH) and tetrahydrofuran (THF)) is reacted with N-succinimidyl- N-methylcarbamate. This results in the formation of the final product, physostigmine, with a yield of 44%. Product yield: 44% Chemistry of the reaction: This is a substitution reaction in which the hydrogen of the phenolic group is replaced with a methylamide (-COONHMe) group. Procedure (steps. reagents \& conditions); The mixture of the phenolic compound (53.0mg,0.24mmol) from the previous reaction containing sodium hydride (NaH)(60%,22mg,0.54mmol) and tetrahydrofuran (THF)) (2 mL ) is stirred at 0C for 5 minutes. Then, N-succinimidyl- N-methylcarbamate is added to this mixture at 0C. The resulting mixture is stirred at room temperature for 1h, quenched with water, and extracted with ethyl acetate (EtOAc). The organic layers are then combined, washed with brine, dried with sodium sulfate (Na2SO4), and concentrated. The residue is finally purified by using flash column chromatography. 6) Step 6: Reaction: (t)-esermethole phenolic compound In this step, the esermethole formed from the previous step is acidified with aqueous hydrobromic acid (HBr), which results in the formation of a phenolic compound. Chemistry of the reaction: The methyl ether (MeO) group on the benzene ring giving rise to a phenolic group (OH). Procedure (steps, reagents \& conditions): Esermethole (92mg,0.40mmol) is dissolved in an aqueous solution of hydrobromic acid ( HBr)(48%,1mL) and stirred at reflux for 19h. The mixture is then cooled and poured into cold water. The acidic solution is basified by using a solution of sodium hydroxide (NaOH) (10%), then extracted with ethyl acetate (EtOAc), dried with sodium sulfate (Na2SO4), and evaporated to dryness. The crude product is used directly in the next step of the reaction without purification. 7) Step 7: phenolic compound Reaction: In the final step of physostigmine synthesis, the mixture of the phenolic compound from the (yield 44\%) previous reaction (also containing sodium hydride (NaH) and tetrahydrofuran (THF)) is reacted with N-succinimidyl- N-methylcarbamate. This results in the formation of the final product, physostigmine, with a yield of 44%. Product yield: 44% Chemistry of the reaction: This is a substitution reaction in which the hydrogen of the phenolic group is replaced with a methylamide (-COONHMe) group. Procedure (steps. reagents \& conditions); The mixture of the phenolic compound (53.0mg,0.24mmol) from the previous reaction containing sodium hydride (NaH)(60%,22mg,0.54mmol) and tetrahydrofuran (THF)) (2 mL ) is stirred at 0C for 5 minutes. Then, N-succinimidyl- N-methylcarbamate is added to this mixture at 0C. The resulting mixture is stirred at room temperature for 1h, quenched with water, and extracted with ethyl acetate (EtOAc). The organic layers are then combined, washed with brine, dried with sodium sulfate (Na2SO4), and concentrated. The residue is finally purified by using flash column chromatography









