these have to do with enthalpy change, i gave 2 examples on how to answer them with the steps involved, at the very bottom pls answer ALL 11 of the questions shown and pls show the steps on how you got it thank u!

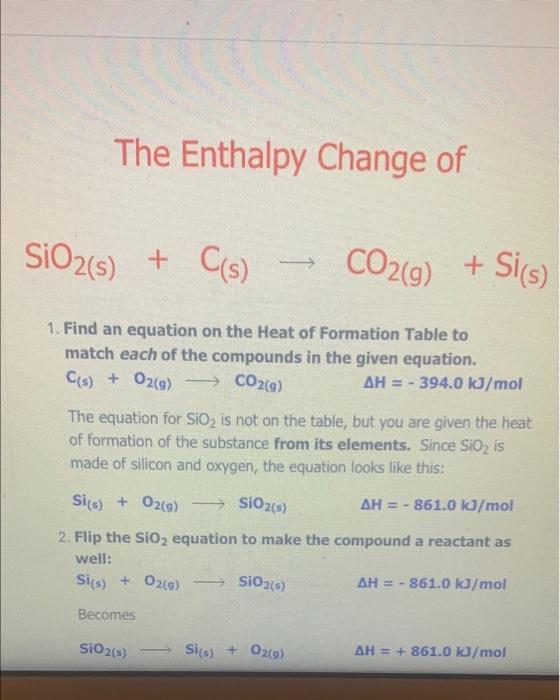
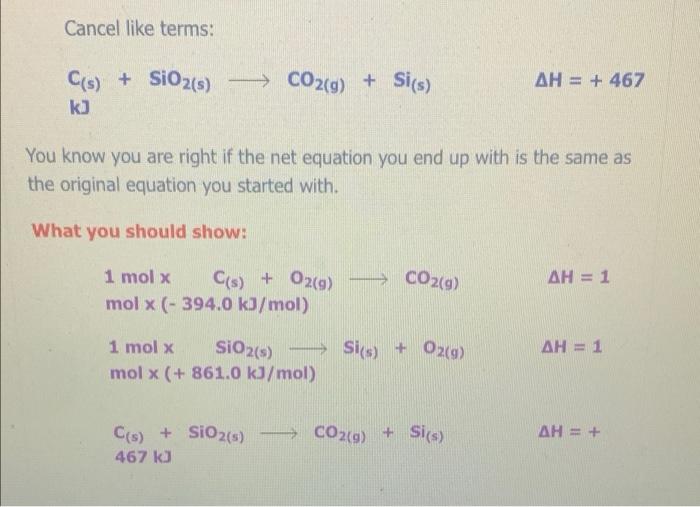
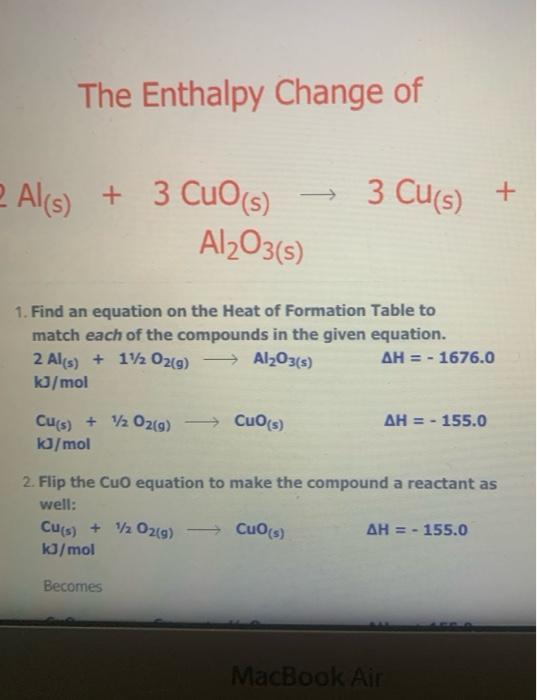
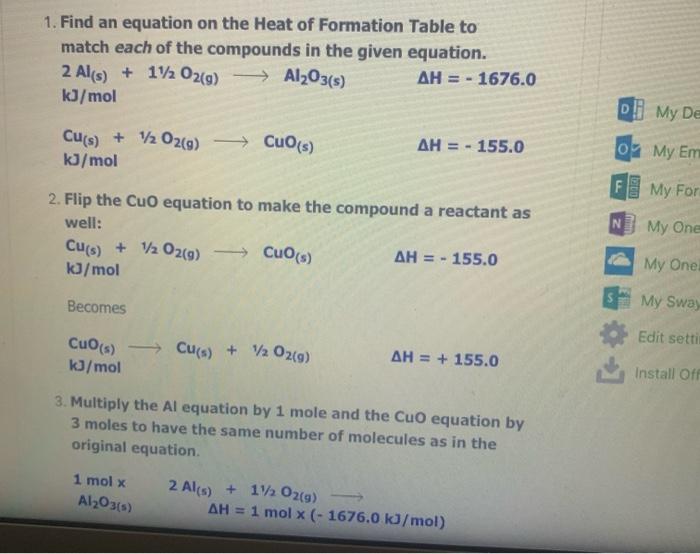
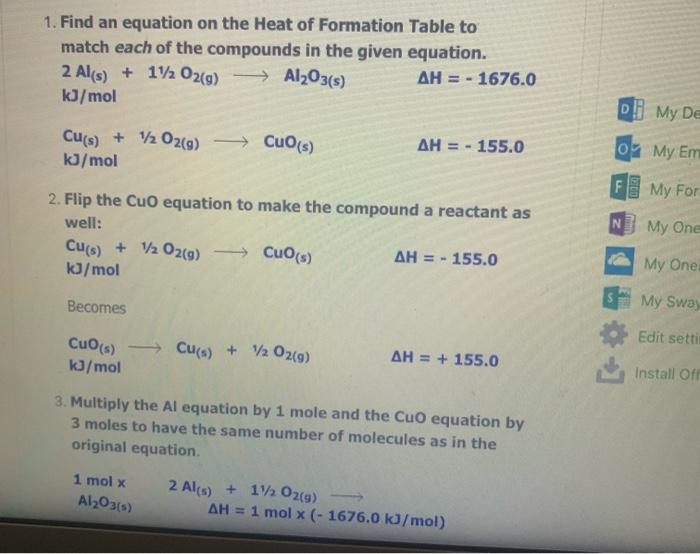
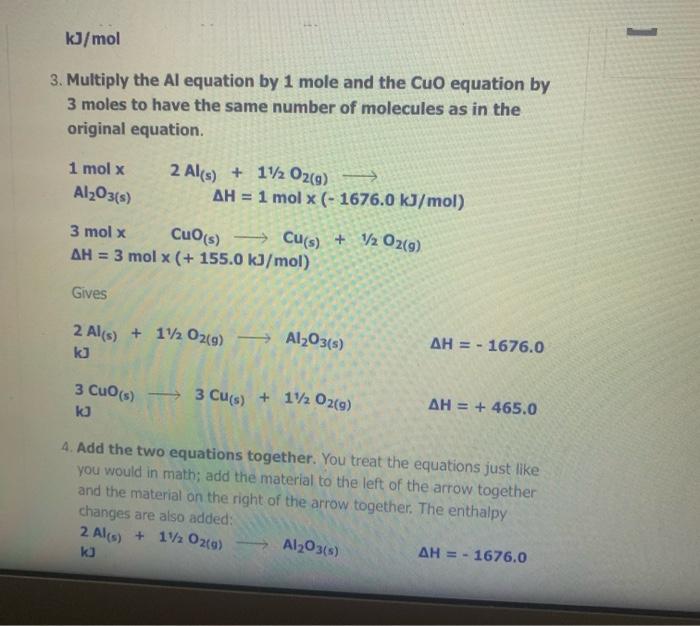
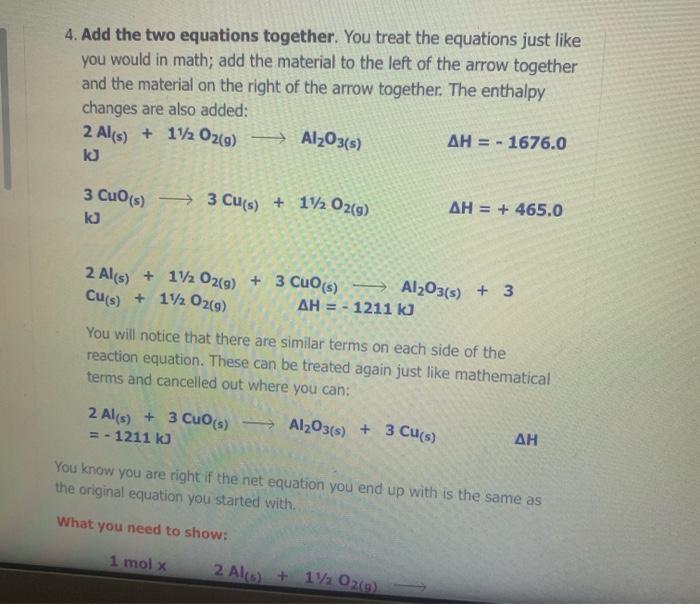
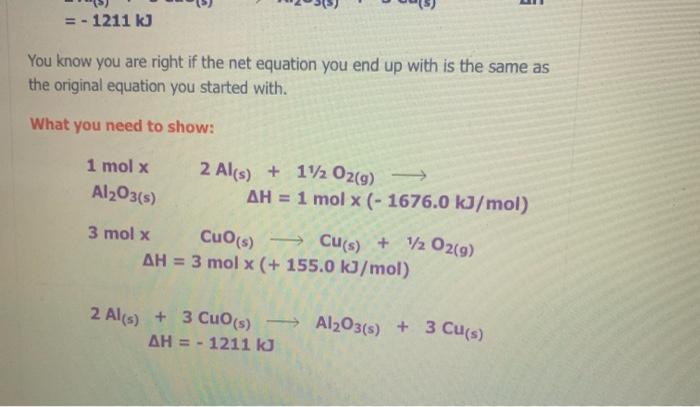
The Enthalpy Change of SiO2(s) + C) + CO2(g) + Sis) 9 1. Find an equation on the Heat of Formation Table to match each of the compounds in the given equation. C(s) + O2(9) -> CO2() AH = - 394.0 kJ/mol The equation for SiO2 is not on the table, but you are given the heat of formation of the substance from its elements. Since Sio, is made of silicon and oxygen, the equation looks like this: Si(s) + 02(9) - SiO2(s) AH = - 861.0 kJ/mol 2. Flip the SiO2 equation to make the compound a reactant as well: Si(s) + O2(g) AH = - 861.0 kJ/mol SIO2) Becomes SiO2(s) Sics) + O2(g) AH = + 861.0 kJ/mol SiO2(s) Si(s) + O2(g) AH = + 861.0 kJ/mol 3. Since there is only one of each molecule in the original equation, multiply each by 1 mole in order to cancel the units: 1 mol x C(s) + O2(g) + CO2(g) AH = 1 mol x(-394.0 kJ/mol) 1 mol x SiO2(s) > Si(s) + O2(g) x(+ 861.0 kJ/mol) AH = 1 mol 4. Add the two equations together. You treat the equations just like you would in math; add the material to the left of the arrow together and the material on the right of the arrow together. The enthalpy changes are also added: C(s) + O2(g) AH = - 394.0 kJ CO2(g) SiO2(s) Si(s) + O2(9) AH = + 861.0 kJ C(s) + O2(g) + SiO2(s) CO2(g) + Si(s) + O2(g) AH = + 467 kJ Cancel like terms: C(s) + SiO2(5) CO2(9) + Si(s) AH = + 467 Cancel like terms: AH = + 467 Cs) + SiO2(s) > CO2(g) + Si(s) k) You know you are right if the net equation you end up with is the same as the original equation you started with. What you should show! 1 mol x C(s) + O2(g) mol x (- 394.0 kJ/mol) CO2(9) AH = 1 AH = 1 1 mol x SiO2(s) - Sis) + O2(g) mol x (+ 861.0 kJ/mol) + Si(s) AH = + C) + SiO2(s) + CO2(9) 467 kJ The Enthalpy Change of 2 e Al(s) + 3 Cuo(s) 3 3 Cu(s) + Al2O3(s) 1. Find an equation on the Heat of Formation Table to match each of the compounds in the given equation. 2 Al(s) + 1/2O2(9) Al2O3(s) AH = - 1676.0 kJ/mol Cuo(s) AH = - 155.0 Cu(s) + 1/2O2(g) kJ/mol 2. Flip the Cuo equation to make the compound a reactant as well: Cu(s) + V2029) CuO (s) AH = - 155.0 kb/mol Becomes MacBook Air 1. Find an equation on the Heat of Formation Table to match each of the compounds in the given equation. 2 Al(s) + 1/2O2(g) - Al2O3(s) AH = - 1676.0 kJ/mol Di My De Cu(s) + 1/2O2(g) Cuo(s) s kJ/mol AH = - 155.0 07 My Em Fl My For N 2. Flip the CuO equation to make the compound a reactant as well: Cu(s) + 12 02(9) - Cuo(s) AH = - 155.0 kJ/mol My One My One Becomes My Sway Edit setti Cuo(s)-> Cu(s) + V2 02(9) kJ/mol AH = + 155.0 Install Off 3. Multiply the Al equation by 1 mole and the Cuo equation by 3 moles to have the same number of molecules as in the original equation 1 mol x Al2O3(s) 2 Al(s) + 112 O2(g) AH = 1 mol x (- 1676.0 kJ/mol) 1. Find an equation on the Heat of Formation Table to match each of the compounds in the given equation. 2 Al(s) + 1/2O2(g) - Al2O3(s) AH = - 1676.0 kJ/mol Di My De Cu(s) + 1/2O2(g) Cuo(s) s kJ/mol AH = - 155.0 07 My Em Fl My For N 2. Flip the CuO equation to make the compound a reactant as well: Cu(s) + 12 02(9) - Cuo(s) AH = - 155.0 kJ/mol My One My One Becomes My Sway Edit setti Cuo(s)-> Cu(s) + V2 02(9) kJ/mol AH = + 155.0 Install Off 3. Multiply the Al equation by 1 mole and the Cuo equation by 3 moles to have the same number of molecules as in the original equation 1 mol x Al2O3(s) 2 Al(s) + 112 O2(g) AH = 1 mol x (- 1676.0 kJ/mol) 1 kJ/mol 3. Multiply the Al equation by 1 mole and the Cuo equation by 3 moles to have the same number of molecules as in the original equation 1 mol x Al2O3(s) 2 Al(s) + 1/2O2(g) AH = 1 mol x (- 1676.0 kJ/mol) 3 mol x Cuo(s) - Cu(s) AH = 3 mol x (+ 155.0 kJ/mol) + 12 02(9) Gives 2 Al(s) + 11/2O2(g) kJ Al2O3(s) AH = - 1676.0 3 Cuo(s) ka 3 Cu(s) + 1/2O2(9) AH = + 465.0 4. Add the two equations together. You treat the equations just like you would in math; add the material to the left of the arrow together and the material on the right of the arrow together. The enthalpy changes are also added: 2 Al(s) + 1V2 O2(a) AH = - 1676.0 ka Al2O3(s) 4. Add the two equations together. You treat the equations just like you would in math; add the material to the left of the arrow together and the material on the right of the arrow together. The enthalpy changes are also added: 2 Al(s) + 1/2O2(g) AlzO3(s) AH = - 1676.0 kJ 3 Cuo(s) 3 Cu(s) + 1/ 02(g) AH = + 465.0 kJ 2 Al(s) Cu(s) + 1/2O2(g) + 3 CuO (s) Al2O3(s) + 3 + 112 O2(g) AH = - 1211 kJ You will notice that there are similar terms on each side of the reaction equation. These can be treated again just like mathematical terms and cancelled out where you can: 2 Al(s) + 3 Cuo(s) - 1211 ka -Al2O3(s) + 3 Cu(s) - AH You know you are right if the net equation you end up with is the same as the original equation you started with What you need to show: 1 molx 2 Ale 11. Ozo) = - 1211 ) You know you are right if the net equation you end up with is the same as the original equation you started with What you need to show: 1 mol x Al2O3(s) 2 Al(s) + 1/2O2(g) -> - AH = 1 mol x (- 1676.0 kJ/mol) 3 mol x Cuo(s) - Cu(s) + 1/2O2(9) AH = 3 mol x (+ 155.0 kJ/mol) 2 Al(s) + 3 Cuo(s) -> Al2O3(s) + 3 Cu(s) AH = - 1211 kJ 9 > 2 SO3 (9) H2SO4 (1) s > 9 1.H200 H2O(9) 2.2 SO2 (g) + O2 (g) 3. SO3 (9) + H200) - 4.2 HI(g) -> H2 (g) H2 (g) + 12 (5) 5.4 NH3 (9) + 5 O2 (9) 9 + 5 O2 (9) > 4 NO(g) + 6 H20(1) 6. CH4 (g) + 2O2 (9) CO2 (g) + 2 H2O(g) + 7. CH4 (g) + 2 02 (9) CO2 (g) + 2 H2O(1) 8. C3H8 (9) + 5 O2 (9) > 3 CO2 (9) + 4 H20() 9.2 Al(s) + Fe2O3 (s) > Al2O3 (s) + 2 Fe(s) where AHF for Fe2O3 (s) = -823 kJ/mol 10. SiO2 (s) + C(s) => CO2 (g) + Si(s) where AHp for SiO2 (s) = -861 kJ/mol 11. P4010 (s) + 6 H2O(1) -> 4 H3PO4 (5) where AHF for P4010 (s) = -3024 kJ/mol and AH for H3PO4 (s) = -1273 kJ/mol 9 (