Answered step by step
Verified Expert Solution
Question
1 Approved Answer
These two questions go together, so thank you for answering them both. I really need 5, but I can't post 5 alone as a question,
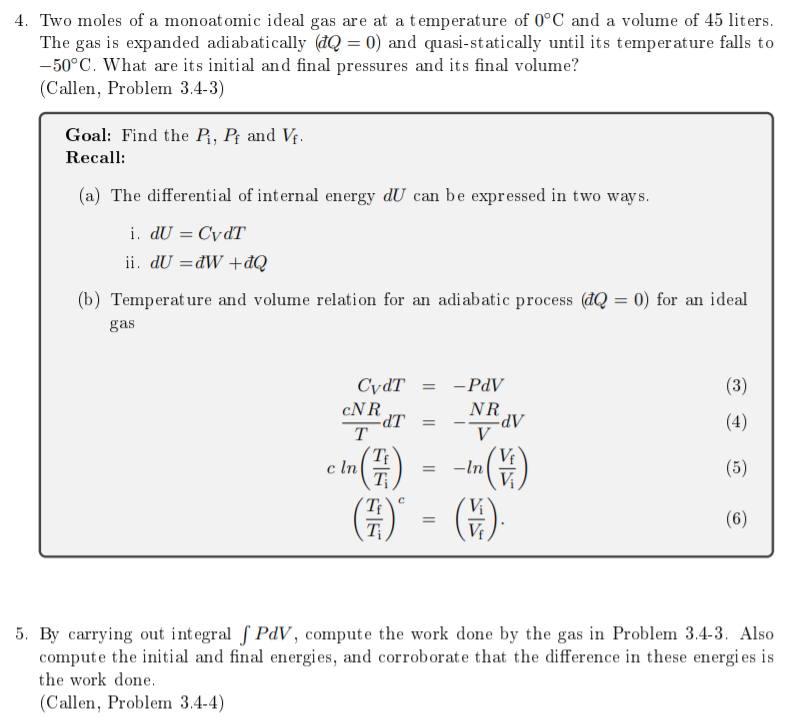
These two questions go together, so thank you for answering them both. I really need 5, but I can't post 5 alone as a question, it would make no sense. ANYWAY - The Callen book solutions given are incomplete/wrong. 4 needs the INITIAL and FINAL pressures and final volume and ALL answers already posted on Chegg have different solutions!!! Eeek. 5 the work done needs to match the difference in energies. In Chegg's solutions they do not. Please help me understand this problem. Thank you!
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


