Answered step by step
Verified Expert Solution
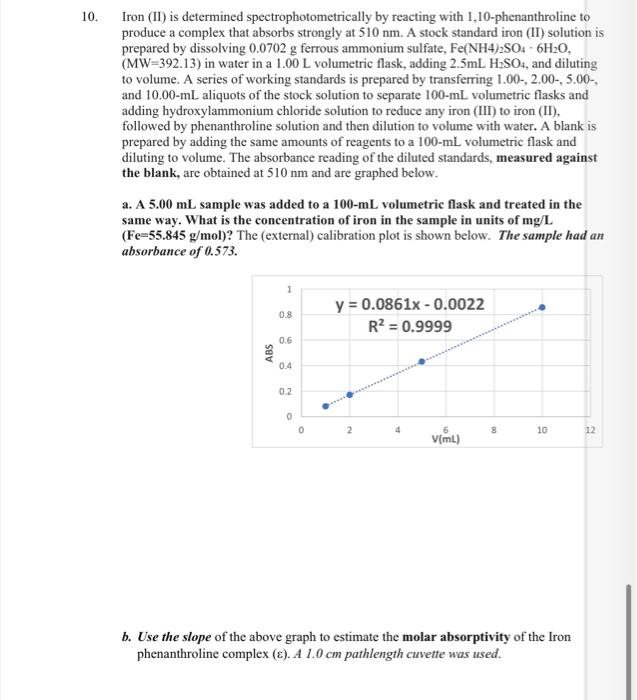
Question
1 Approved Answer
This is as given. Requested extra information is not available in the question. Iron (II) is determined spectrophotometrically by reacting with 1,10-phenanthroline to produce a
This is as given. Requested extra information is not available in the question.
Iron (II) is determined spectrophotometrically by reacting with 1,10-phenanthroline to produce a complex that absorbs strongly at 510nm. A stock standard iron (II) solution is prepared by dissolving 0.0702g ferrous ammonium sulfate, Fe(NH4)2SO46H2O, (MW=392.13) in water in a 1.00L volumetric flask, adding 2.5mLH2SO4, and diluting to volume. A series of working standards is prepared by transferring 1.00,2.005.00, and 10.00mL aliquots of the stock solution to separate 100mL volumetric flasks and adding hydroxylammonium chloride solution to reduce any iron (III) to iron (II), followed by phenanthroline solution and then dilution to volume with water. A blank is prepared by adding the same amounts of reagents to a 100mL volumetric flask and diluting to volume. The absorbance reading of the diluted standards, measured against the blank, are obtained at 510nm and are graphed below. a. A 5.00mL sample was added to a 100mL volumetric flask and treated in the same way. What is the concentration of iron in the sample in units of mg/L ( Fe=55.845g/mol )? The (external) calibration plot is shown below. The sample had an absorbance of 0.573. b. Use the slope of the above graph to estimate the molar absorptivity of the Iron Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


