Question
This is from one of my organic chemistry lectures where the question was to order the following molecules in order of stability. My professor said
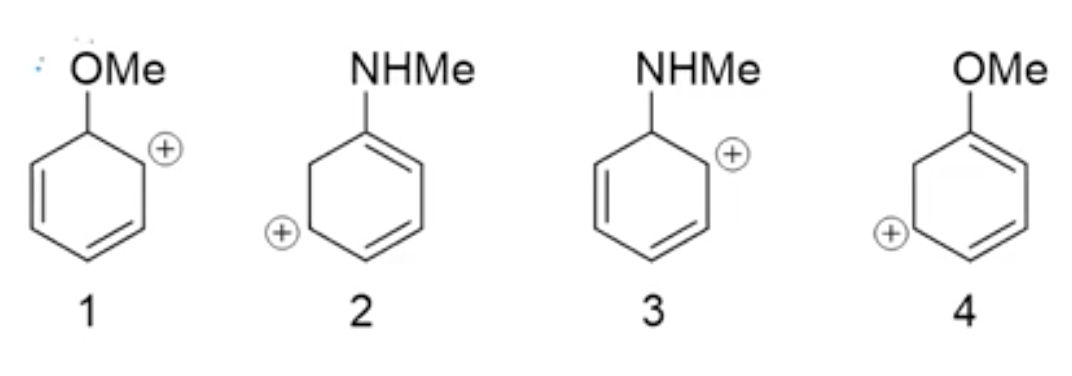
This is from one of my organic chemistry lectures where the question was to order the following molecules in order of stability. My professor said that (1) and (3) have no resonance structures which confused me since I thought they have resonance structures. Consider just molecule (1), can't the electron in the pi bond of the bottom carbon move up and to the left to form a pi bond with the two right-most carbons creating a positive charge on the bottom carbon as shown in the picture. Why could this not be a resonance structure? (Don't need to answer the stability question, just the reason why molecules (1) and (3) have no resonance structures)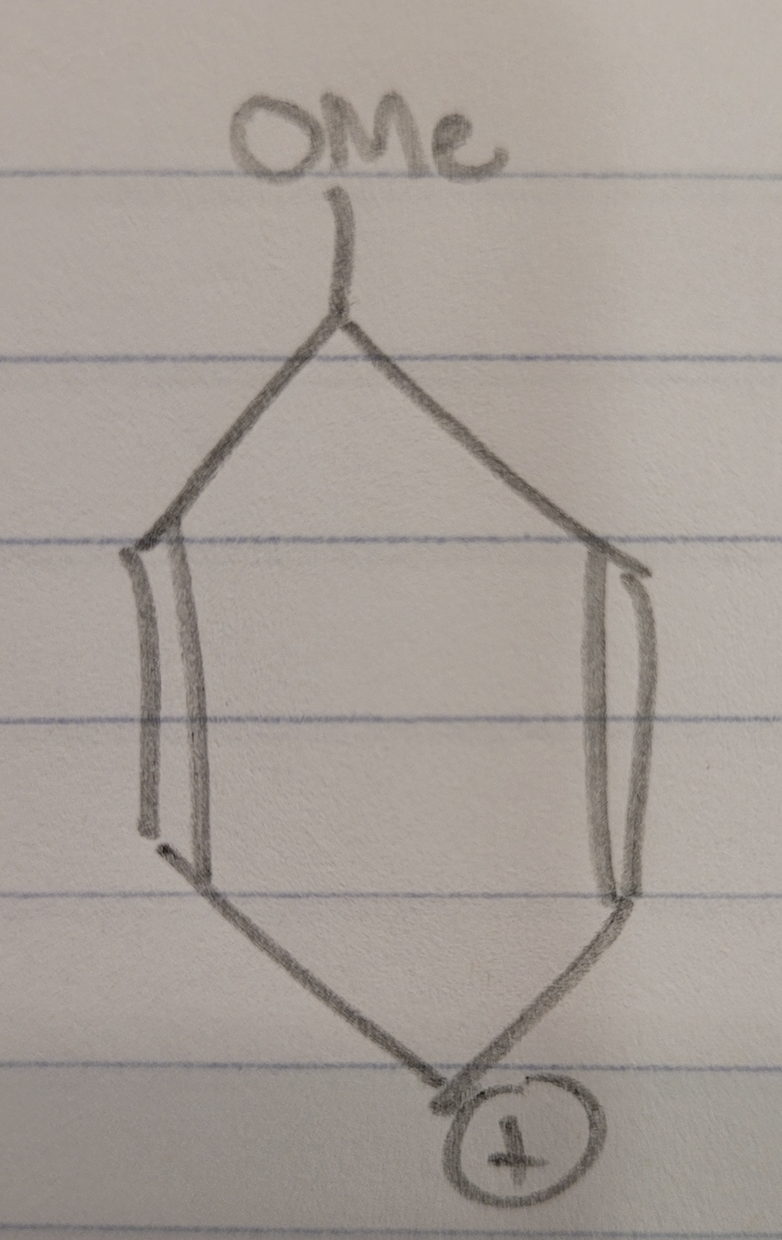
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


