Answered step by step
Verified Expert Solution
Question
1 Approved Answer
This is the whole IDP diagram that we made : All the value of temperature, pressure, mol fraction those stuff can just assume. So just
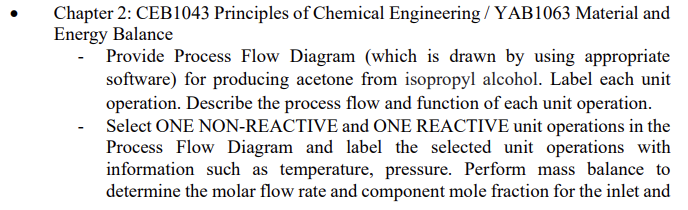

This is the whole IDP diagram that we made :
All the value of temperature, pressure, mol fraction those stuff can just assume. So just choose a non-reactive system and reactive system to calculate. I would recommend to do the calculation at the H1(non-reactive) and CRx1(reactive).
Chapter 2: CEB1043 Principles of Chemical Engineering / YAB1063 Material and Energy Balance - Provide Process Flow Diagram (which is drawn by using appropriate software) for producing acetone from isopropyl alcohol. Label each unit operation. Describe the process flow and function of each unit operation. Select ONE NON-REACTIVE and ONE REACTIVE unit operations in the Process Flow Diagram and label the selected unit operations with information such as temperature, pressure. Perform mass balance to determine the molar flow rate and component mole fraction for the inlet and outlet streams of the selected unit operations. Provide calculations for the mass balance and state all assumptions in your calculation with references. Perform energy balance for the same selected ONE NON-REACTIVE and ONE REACTIVE unit operations. Provide calculations for the energy balance and state all assumptions in your calculation with references. Visual Paradigm Online Free Edition S7 Hydrogen Isopropyl alcohol with water S6 S1 S2 S3 S4 S5 Absorber --- Sep1 M1 H1 C1 CRx1 S13 Waste water S10 S8 59 T1 S11 Acetone T2 S12 Visual Paradigm Online Free Edition
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


