Answered step by step
Verified Expert Solution
Question
1 Approved Answer
This question has multiple parts. Work all the parts to get the most points. a If you mix equal volumes of 0.1MHCl and 0.2M PIPES
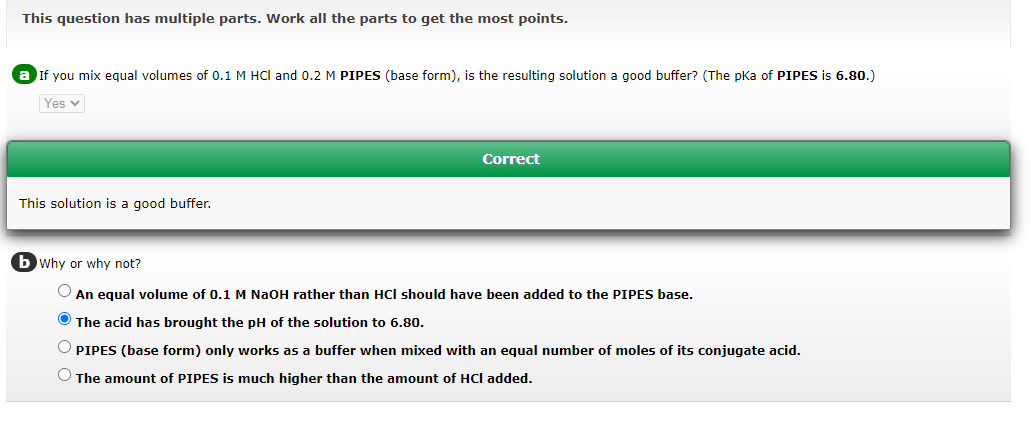

This question has multiple parts. Work all the parts to get the most points. a If you mix equal volumes of 0.1MHCl and 0.2M PIPES (base form), is the resulting solution a good buffer? (The pKa of PIPES is 6.80.) Correct This solution is a good buffer. b Why or why not? An equal volume of 0.1MNaOH rather than HCl should have been added to the PIPES base. The acid has brought the pH of the solution to 6.80. PIPES (base form) only works as a buffer when mixed with an equal number of moles of its conjugate acid. The amount of PIPES is much higher than the amount of HCl added. If equal volumes of 0.1MHCl and 0.2MHEPES (base form) are mixed together. The pKa of HEPES is 7.55. Which of the following statements about the resulting solution are correct? (Select all that apply.) This solution is a good buffer. The ratio of [conjugate base]/[conjugate acid] is [0.2M]/[0.1M]. The ratio of [conjugate base]/[conjugate acid] is [0.05M]/[0.05M]. The HCl will protonate 0.1M of the HEPES base form. This solution is too acidic to be a buffer
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


