Question
Based on Molecular Orbital theory, determine the bond order of the following. Also indicate if the molecule is paramagnetic or diamagnetic. (a) 02px Bond

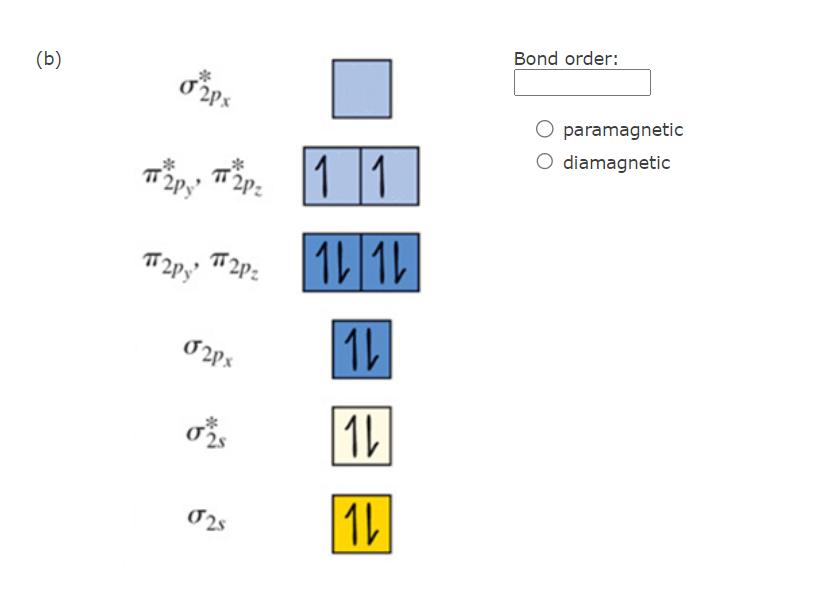
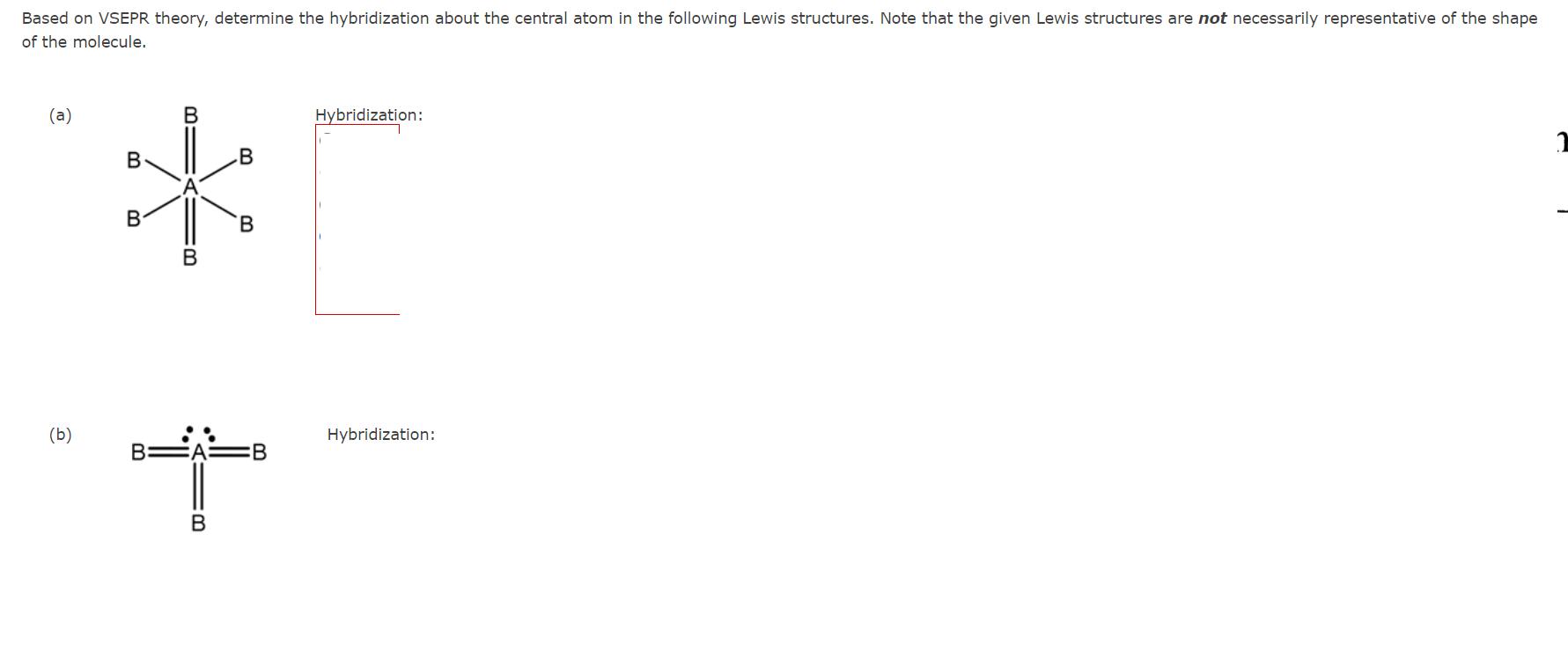
Based on Molecular Orbital theory, determine the bond order of the following. Also indicate if the molecule is paramagnetic or diamagnetic. (a) 02px Bond order: py 02px #2P: 025 paramagnetic O diamagnetic 025 1 (b) T2Py T2P2 T2P2 T2Py 024x 11 12 12 1L 1 025 1b Bond order: paramagnetic diamagnetic Based on VSEPR theory, determine the hybridization about the central atom in the following Lewis structures. Note that the given Lewis structures are not necessarily representative of the shape of the molecule. (a) B B Hybridization: B ** B B B (b) Hybridization: B=A= B B 1
Step by Step Solution
3.30 Rating (150 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Calculus
Authors: Ron Larson, Bruce H. Edwards
10th Edition
1285057090, 978-1285057095
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



