Question
took the ir spectrum of their crude product and their purified product. Do you believe that the purified product could be the one they
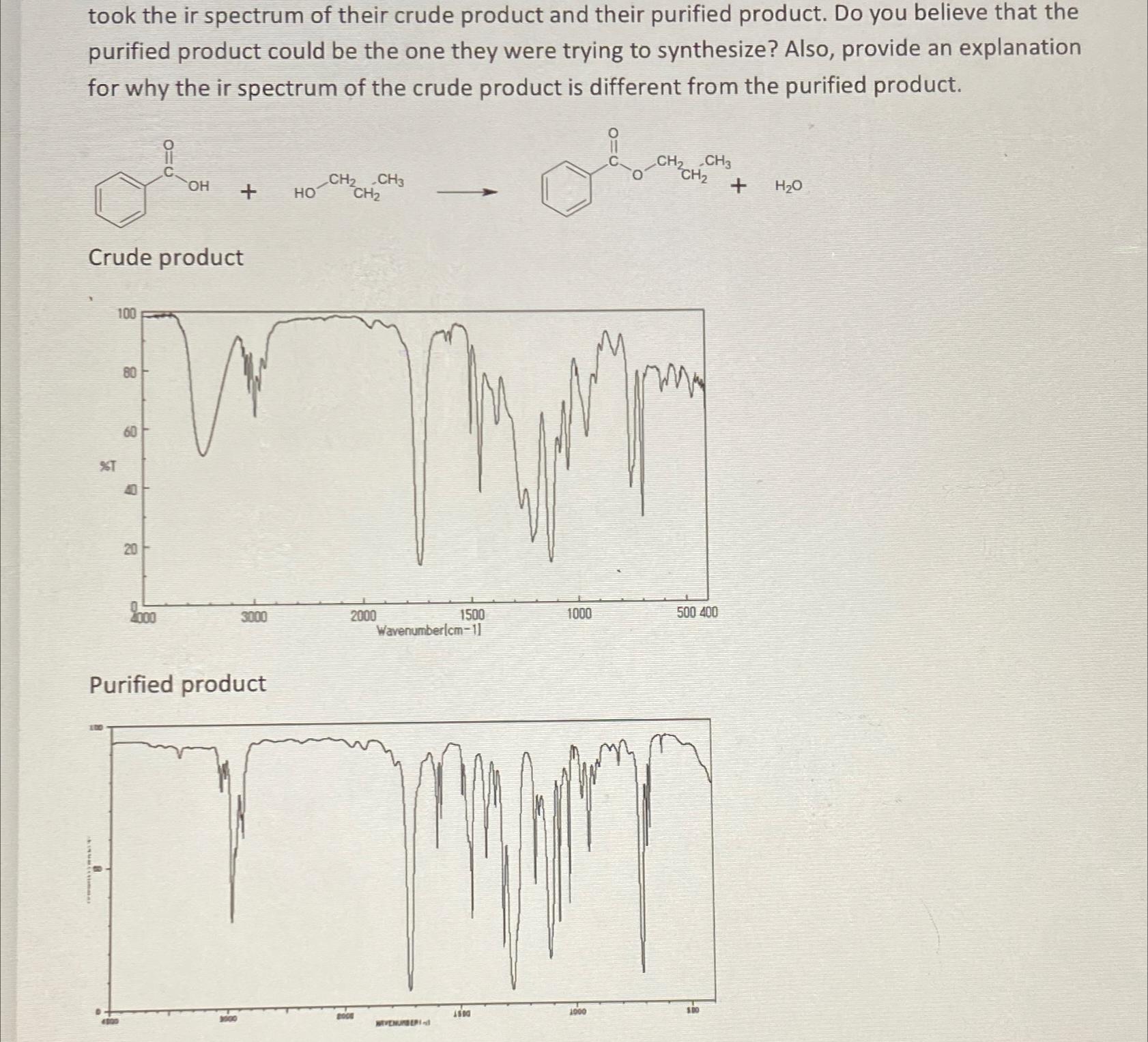
took the ir spectrum of their crude product and their purified product. Do you believe that the purified product could be the one they were trying to synthesize? Also, provide an explanation for why the ir spectrum of the crude product is different from the purified product. 100 %T Crude product 80 60 8 C 2000 OH + 3000 Purified product - CH CH3 CH 2000 2008 1500 Wavenumber(cm-11 WEVENURBERI -d 1500 CH CH3 Obavan CH 1000 1000 500 400 $.00 + HO
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Solutions Step 1 There is difference between the purified product and crude product and those ir spe...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Business Ethics Ethical Decision Making & Cases
Authors: O. C. Ferrell, John Fraedrich, Linda Ferrell
8th Edition
1439042233, 978-1439042236
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



