Question: Topic: Physics: Statistical Mechanics. Instructions: Answer the Problems below and Show Complete Solution. FIRST and SECOND LAWS OF THERMODYNAMICS Introductory Statistical Mechanics Read University Physics
Topic: Physics: Statistical Mechanics.
Instructions: Answer the Problems below and Show Complete Solution.
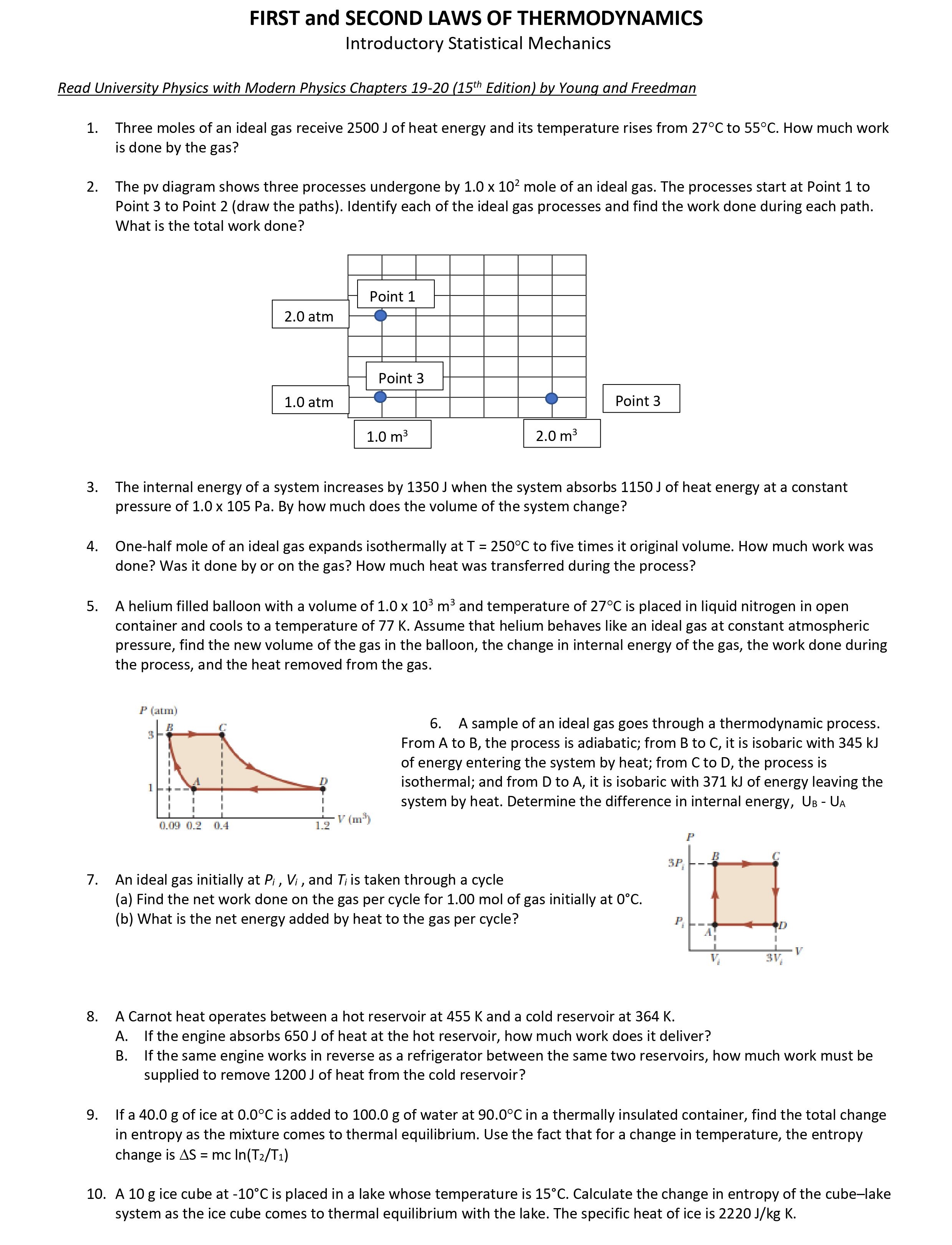

FIRST and SECOND LAWS OF THERMODYNAMICS Introductory Statistical Mechanics Read University Physics With Modern Physics Chapters 19-20 (15\"7 Edition) by Young and Freedman 1. 2. 10. Three moles of an ideal gas receive 2500] of heat energy and its temperature rises from 27C to 55C. How much work is done by the gas? The pv diagram shows three processes undergone by 1.0 x 102 mole of an ideal gas. The processes start at Point 1 to Point 3 to Point 2 (draw the paths). Identify each of the ideal gas processes and find the work done during each path. What is the total work done? The internal energy of a system increases by 1350] when the system absorbs 115OJ of heat energy at a constant pressure of 1.0 x 105 Pa. By how much does the volume of the system change? One-half mole of an ideal gas expands isothermally at T = 250C to five times it original volume. How much work was done? Was it done by or on the gas? How much heat was transferred during the process? A helium filled balloon with a volume of 1.0 x 103 m3 and temperature of 27C is placed in liquid nitrogen in open container and cools to a temperature of 77 K. Assume that helium behaves like an ideal gas at constant atmospheric pressure, find the new volume of the gas in the balloon, the change in internal energy of the gas, the work done during the process, and the heat removed from the gas. FEE\": r: 6. A sample of an ideal gas goes through a thermodynamic process. From A to B, the process IS adiabatic; from B to C, It IS Isobarlc With 345 k] i of energy entering the system by heat; from C to D, the process is 1 L D isothermal; and from D to A, it is isobaric with 371 k] of energy leaving the I : i : system by heat. Determine the difference in internal energy, Us UA ".119 \".2 9.4 L2 V [[115]. P 3P. _ a r An ideal gas initially at Pi, Vi , and Ti is taken through a cycle (a) Find the net work done on the gas per cycle for 1.00 mol of gas initially at 0C. (b) What is the net energy added by heat to the gas per cycle? P. _A n A Carnot heat operates between a hot reservoir at 455 K and a cold reservoir at 364 K. A. If the engine absorbs 650] of heat at the hot reservoir, how much work does it deliver? B. Ifthe same engine works in reverse as a refrigerator between the same two reservoirs, how much work must be supplied to remove 1200] of heat from the cold reservoir? If a 40.0 g of ice at 0.0C is added to 100.0 g of water at 90.0C in a thermally insulated container, find the total change in entropy as the mixture comes to thermal equilibrium. Use the fact that for a change in temperature, the entropy change is AS = mcln(T2/T1) A 10 g ice cu be at -10C is placed in a lake whose temperature is 15C. Calculate the change in entropy of the cubelake system as the ice cube comes to thermal equilibrium with the lake. The specific heat of ice is 2220 J/kg K. 11. The figure shows a reversible cycle through which 1.00 mol of a monatomic ideal gas is 1; taken. Assume that p = 2pc, V= 2V0, po = 1.01 x 105 Pa, and V0 = 0.0225 m3. Calculate H P (a) the work done during the cycle (b) the energy added as heat during stroke abc, and (c) the efficiency of the cycle. (cl) What is the efficiency of a Carnot engine operating between the highest and lowest '3 temperatures that occur in the cycle? (e) Is this greater than or less than the efficiency calculated in (c)? Prcssurc Volume
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


