Answered step by step
Verified Expert Solution
Question
1 Approved Answer
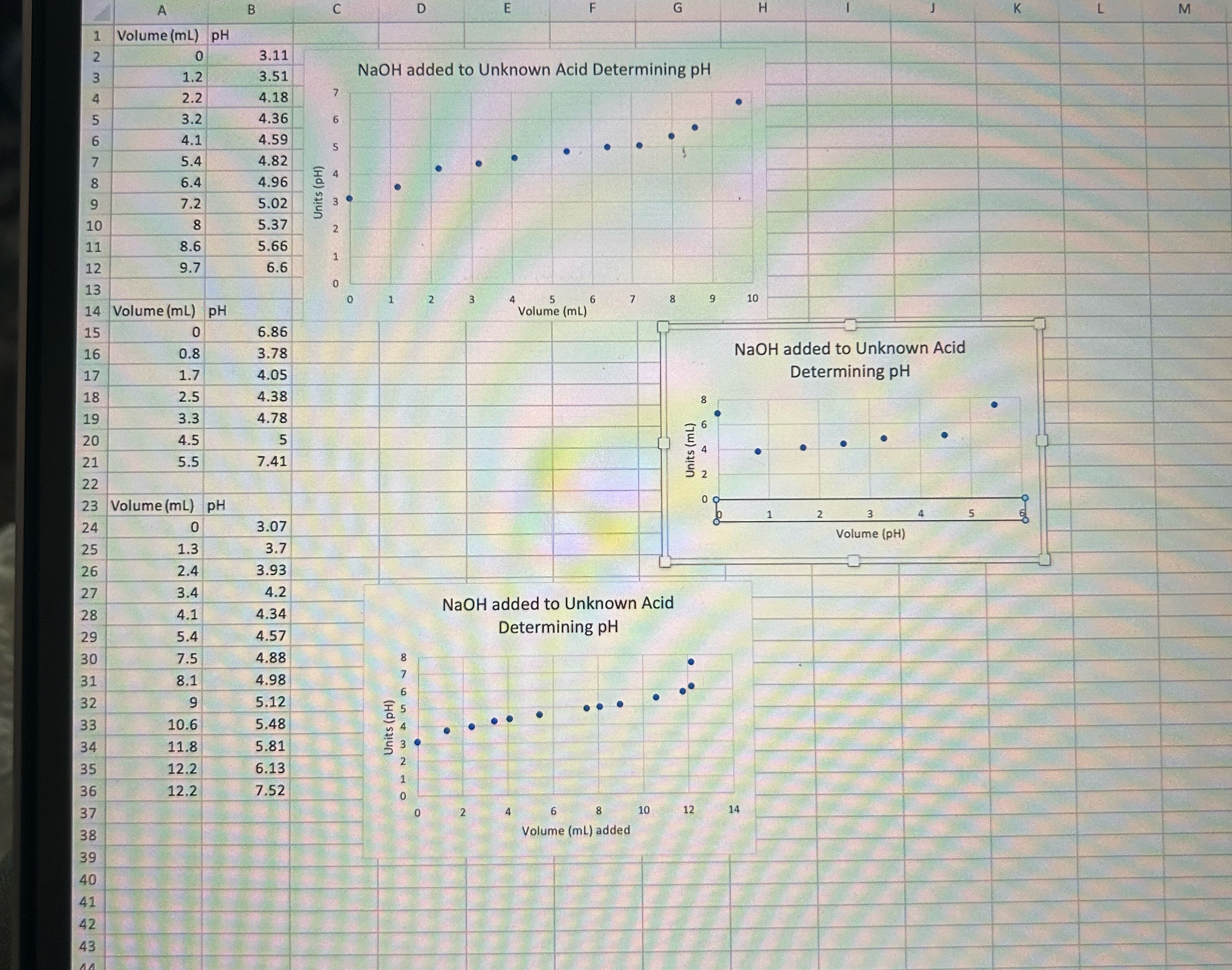
Trial 1 : Mass of KHP: 0 . 3 5 0 g Volume of 0 . 1 M NaOH used to reach equilibrium: 1 7
Trial :
Mass of KHP: g
Volume of M NaOH used to reach equilibrium: mL
Trial :
Mass of KHP: g
Volume of M NaOH used to reach equilibrium: mL
Trial :
Mass of KHP:
Volume of M NaOH used to reach equilibrium: mL
Determine volume of NaOH at equivalence point for each trial?
Determine volume of NaOH at
equivalence point for each trial?
PKa for each trial?
Ka for each trial?
Average Ka
Standard deviation Ka
RSD of Ka
mL of unknown acid in eaxh trial.
Find average concentration of NaOH M in each trial?
Volume of NaOH at equivalence point?
Concentration of unknown acid M
Average concentration?
Standard deviation?
RSD
Please help me figure out how to solve these problems!! The excel sheet goes in order from trial top to trial bottom Thank you!!
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


