Answered step by step
Verified Expert Solution
Question
1 Approved Answer
(Turns,2013) 5.13 Consider the production of nitric oxide (NO) in the following combustion systems using the Zeldovich mechanism given in Eq. 5.7. (Also see the
(Turns,2013) 5.13 Consider the production of nitric oxide (NO) in the following combustion systems using the Zeldovich mechanism given in Eq. 5.7. (Also see the Chapter 4, Examples 4.3 and 4.4.). In each case, assume that the environment is well mixed; O, O2 and N2 are in their equilibrium compositions (given); the N atoms are in steady state; the temperature is fixed (given) and the reactions reverses are negligible. Calculate the NO concentration in parts per million (ppm) and the ratio between the value of the NO concentration predicted by the kinetics chemical and that provided by chemical equilibrium. Comment on the validityto neglect the reverse reactions. Do your results make sense?
A. Consider the operation of a gas turbine in a thermoelectric generation plant, operating without the presence of emission control measures. The primary zone of the combustion chamber of this gas turbine burns with equivalence ratio of 1.0. The average residence time of the products of combustion under the following conditions is 7 ms.
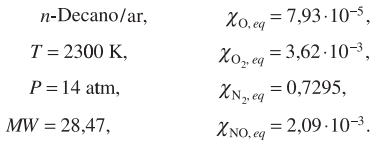
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


