Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Two consecutive reactions occur according to the reaction scheme shown below: Ak1Ik2P The values for each of the rate constants are: k1=0.12s1 and k2=0.14s1. (a)
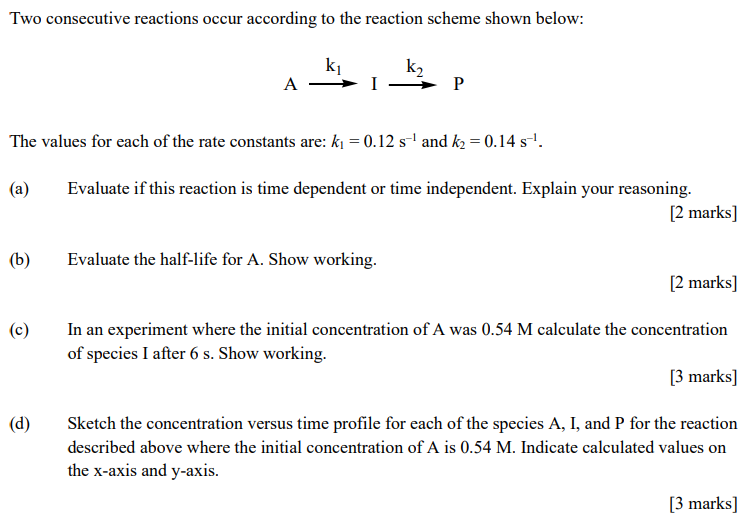 Two consecutive reactions occur according to the reaction scheme shown below: Ak1Ik2P The values for each of the rate constants are: k1=0.12s1 and k2=0.14s1. (a) Evaluate if this reaction is time dependent or time independent. Explain your reasoning. [2 marks ] (b) Evaluate the half-life for A. Show working. [2 marks ] (c) In an experiment where the initial concentration of A was 0.54M calculate the concentration of species I after 6s. Show working. [3 marks ] (d) Sketch the concentration versus time profile for each of the species A, I, and P for the reaction described above where the initial concentration of A is 0.54M. Indicate calculated values on the x-axis and y-axis. [3 marks]
Two consecutive reactions occur according to the reaction scheme shown below: Ak1Ik2P The values for each of the rate constants are: k1=0.12s1 and k2=0.14s1. (a) Evaluate if this reaction is time dependent or time independent. Explain your reasoning. [2 marks ] (b) Evaluate the half-life for A. Show working. [2 marks ] (c) In an experiment where the initial concentration of A was 0.54M calculate the concentration of species I after 6s. Show working. [3 marks ] (d) Sketch the concentration versus time profile for each of the species A, I, and P for the reaction described above where the initial concentration of A is 0.54M. Indicate calculated values on the x-axis and y-axis. [3 marks] Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


