Question: Unisim Assignment Reactor Steam Phare Separator Othe Ful Problem Statement You are going to use is to simulate a steady state process to make 150.000


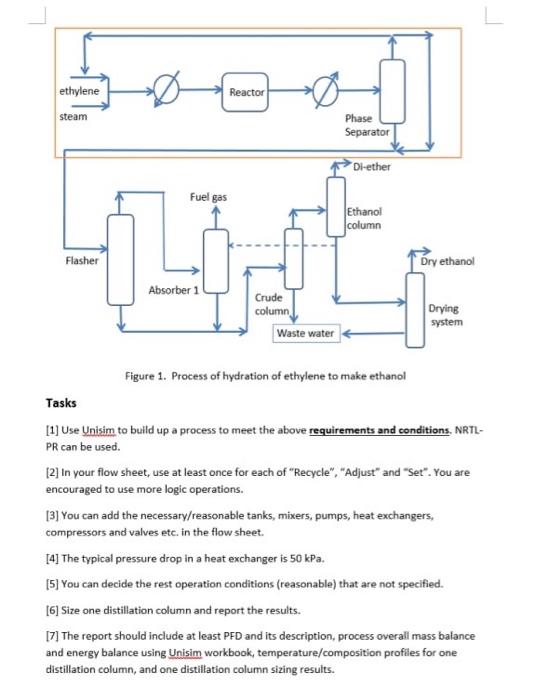
Unisim Assignment Reactor Steam Phare Separator Othe Ful Problem Statement You are going to use is to simulate a steady state process to make 150.000 tom of dry ethanol per year from ethylene hydration. The main reactions GH+H.0GHOH Under the reaction condition, ethanol can for dethyl ether (d-ather) as shown below side reaction, which has 16 electivity. The overall ethylene cometion is 20% 2+1,0 The procese scheme is shown in Figure 1. Freshethylene contain that it will with the recycled ethylene before led to the exter which ist 585 and 20 Steam's another rectant. The moleflowrate ratio of ethylene fred and team is about inter Ethanol column 11- Flasher Oryahan Abs1 Grude column Walte water Drying system Using chilled water, the stream from the reactor will be condensed to 20 before entering Phane Separater. The vapour stream from the phone separator contains mainly ethylene About 95% of the vapour will be recycled to the reactor and the rest will join the liquid to a Father operating tatmand 10 The vapor from the asher will be sent to an Aberber to absorb the heavy organies. The vert from the absorber withered as fuelos. About 20% of the ethanol/water misture from the Ethanol Column will be used as the solvent for this absorber. Absorber would be more efficient tower temperatur The Biquid stream from the flashat and the absorber contain mainly than water and do ther. It will be aparated in a rude Calon first to remove 95. recovery of water from the bottom which contains less than 0.01 molekuthanol The distillate from the crude column will be further purified to remove most of diether in the Shanel Calum where the bottom contains less than 0.001 mole diethyl ether and the top contains than 0.1 molethanol. You may need high reflux ratio and tray for this com The bottom ethanol stien till contains water due to entence of a citrope. It can be further separated in an Stem by other methods tuch as extractive datation doption or mame to get diyethanol Figure 1. Process of hydration of ethylene to make ethanol Tasks 1 Use Unito build up a process to meet the above requirements and conditions PR can be vie 27 in your flow sheet, at once for each othecycle","dur" und "ut". You we encouraged to morogle operations You can add the recevresele tanks, sumpsest exchangers compressions and set in the flow shot 14 The typical pressure drop in a heat exchanger SOP 15. You can decide the rest operation conditions reasonable that are not specified, 16 See one distillation column and report the | The report should include at least PFO and its description process overall mass balance and enery balance using om workbook, temperature composition profiles for one distillation column, and one diutilation columnsing results Unisim Assignment Problem Statement You are going to use Unisim to simulate a steady state process to make 150,000 tons of dry ethanol per year from ethylene hydration. The main reaction is CzHe+ H20 C3H5OH Under the reaction conditions, ethanol can form diethyl ether (di-ether) as shown below side reaction, which has 16% selectivity. The overall ethylene conversion is 20%. 2CH=CH-CH20O+ H2O The process scheme is shown in Figure 1. Fresh ethylene contains 0.2% ethane. It will mix with the recycled ethylene before fed to the Reactor which is at 585K and 80 atm. Steam is another reactant. The mole flowrate ratio of ethylene feed and steam is about 1:1 to enter reactor. Using chilled water, the stream from the reactor will be condensed to 20C before entering a Phase Separator. The vapour stream from the phase separator contains mainly ethylene. About 95% of the vapour will be recycled to the reactor and the rest will join the liquid to a Flasher operating at 3 atm and 10 C. The vapour from the flasher will be sent to an Absorber to absorb the heavy organics. The vent from the absorber will be used as fuel gas. About 20% of the ethanol/water mixture from the Ethanol Column will be used as the solvent for this absorber. Absorber would be more efficient at lower temperature. The liquid streams from the flasher and the absorber contain mainly ethanol, water and di- ether. It will be separated in a Crude Column first to remove 95% (recovery) of water from the bottom which contains less than 0.01 molex ethanol. The distillate from the crude column will be further purified to remove most of di-ether in the Ethanol Column where the bottom contains less than 0.001 mole diethyl ether and the top contains less than 0.1 mole% ethanol. You may need high reflux ratio and trays for this column. The bottom ethanol stream still contains water due to existence of azeotrope. It can be further separated in a Drying System by other methods such as extractive distillation, adsorption or membrane to get dry ethanol ethylene Reactor steam Phase Separator Di-ether Fuel gas Ethanol column Flasher Dry ethanol Absorber Crude column Waste water Drying system Figure 1. Process of hydration of ethylene to make ethanol Tasks [1] Use Unisim to build up a process to meet the above requirements and conditions. NRTL- PR can be used [2] In your flow sheet, use at least once for each of "Recycle", "Adjust" and "Set". You are encouraged to use more logic operations. [3] You can add the necessary/reasonable tanks, mixers, pumps, heat exchangers, compressors and valves etc. in the flow sheet. [4] The typical pressure drop in a heat exchanger is 50 kPa. [5] You can decide the rest operation conditions (reasonable) that are not specified. [6] Size one distillation column and report the results. [7] The report should include at least PFD and its description, process overall mass balance and energy balance using Unisim workbook, temperature/composition profiles for one distillation column, and one distillation column sizing results. Unisim Assignment Reactor Steam Phare Separator Othe Ful Problem Statement You are going to use is to simulate a steady state process to make 150.000 tom of dry ethanol per year from ethylene hydration. The main reactions GH+H.0GHOH Under the reaction condition, ethanol can for dethyl ether (d-ather) as shown below side reaction, which has 16 electivity. The overall ethylene cometion is 20% 2+1,0 The procese scheme is shown in Figure 1. Freshethylene contain that it will with the recycled ethylene before led to the exter which ist 585 and 20 Steam's another rectant. The moleflowrate ratio of ethylene fred and team is about inter Ethanol column 11- Flasher Oryahan Abs1 Grude column Walte water Drying system Using chilled water, the stream from the reactor will be condensed to 20 before entering Phane Separater. The vapour stream from the phone separator contains mainly ethylene About 95% of the vapour will be recycled to the reactor and the rest will join the liquid to a Father operating tatmand 10 The vapor from the asher will be sent to an Aberber to absorb the heavy organies. The vert from the absorber withered as fuelos. About 20% of the ethanol/water misture from the Ethanol Column will be used as the solvent for this absorber. Absorber would be more efficient tower temperatur The Biquid stream from the flashat and the absorber contain mainly than water and do ther. It will be aparated in a rude Calon first to remove 95. recovery of water from the bottom which contains less than 0.01 molekuthanol The distillate from the crude column will be further purified to remove most of diether in the Shanel Calum where the bottom contains less than 0.001 mole diethyl ether and the top contains than 0.1 molethanol. You may need high reflux ratio and tray for this com The bottom ethanol stien till contains water due to entence of a citrope. It can be further separated in an Stem by other methods tuch as extractive datation doption or mame to get diyethanol Figure 1. Process of hydration of ethylene to make ethanol Tasks 1 Use Unito build up a process to meet the above requirements and conditions PR can be vie 27 in your flow sheet, at once for each othecycle","dur" und "ut". You we encouraged to morogle operations You can add the recevresele tanks, sumpsest exchangers compressions and set in the flow shot 14 The typical pressure drop in a heat exchanger SOP 15. You can decide the rest operation conditions reasonable that are not specified, 16 See one distillation column and report the | The report should include at least PFO and its description process overall mass balance and enery balance using om workbook, temperature composition profiles for one distillation column, and one diutilation columnsing results Unisim Assignment Problem Statement You are going to use Unisim to simulate a steady state process to make 150,000 tons of dry ethanol per year from ethylene hydration. The main reaction is CzHe+ H20 C3H5OH Under the reaction conditions, ethanol can form diethyl ether (di-ether) as shown below side reaction, which has 16% selectivity. The overall ethylene conversion is 20%. 2CH=CH-CH20O+ H2O The process scheme is shown in Figure 1. Fresh ethylene contains 0.2% ethane. It will mix with the recycled ethylene before fed to the Reactor which is at 585K and 80 atm. Steam is another reactant. The mole flowrate ratio of ethylene feed and steam is about 1:1 to enter reactor. Using chilled water, the stream from the reactor will be condensed to 20C before entering a Phase Separator. The vapour stream from the phase separator contains mainly ethylene. About 95% of the vapour will be recycled to the reactor and the rest will join the liquid to a Flasher operating at 3 atm and 10 C. The vapour from the flasher will be sent to an Absorber to absorb the heavy organics. The vent from the absorber will be used as fuel gas. About 20% of the ethanol/water mixture from the Ethanol Column will be used as the solvent for this absorber. Absorber would be more efficient at lower temperature. The liquid streams from the flasher and the absorber contain mainly ethanol, water and di- ether. It will be separated in a Crude Column first to remove 95% (recovery) of water from the bottom which contains less than 0.01 molex ethanol. The distillate from the crude column will be further purified to remove most of di-ether in the Ethanol Column where the bottom contains less than 0.001 mole diethyl ether and the top contains less than 0.1 mole% ethanol. You may need high reflux ratio and trays for this column. The bottom ethanol stream still contains water due to existence of azeotrope. It can be further separated in a Drying System by other methods such as extractive distillation, adsorption or membrane to get dry ethanol ethylene Reactor steam Phase Separator Di-ether Fuel gas Ethanol column Flasher Dry ethanol Absorber Crude column Waste water Drying system Figure 1. Process of hydration of ethylene to make ethanol Tasks [1] Use Unisim to build up a process to meet the above requirements and conditions. NRTL- PR can be used [2] In your flow sheet, use at least once for each of "Recycle", "Adjust" and "Set". You are encouraged to use more logic operations. [3] You can add the necessary/reasonable tanks, mixers, pumps, heat exchangers, compressors and valves etc. in the flow sheet. [4] The typical pressure drop in a heat exchanger is 50 kPa. [5] You can decide the rest operation conditions (reasonable) that are not specified. [6] Size one distillation column and report the results. [7] The report should include at least PFD and its description, process overall mass balance and energy balance using Unisim workbook, temperature/composition profiles for one distillation column, and one distillation column sizing results
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


