Answered step by step
Verified Expert Solution
Question
1 Approved Answer
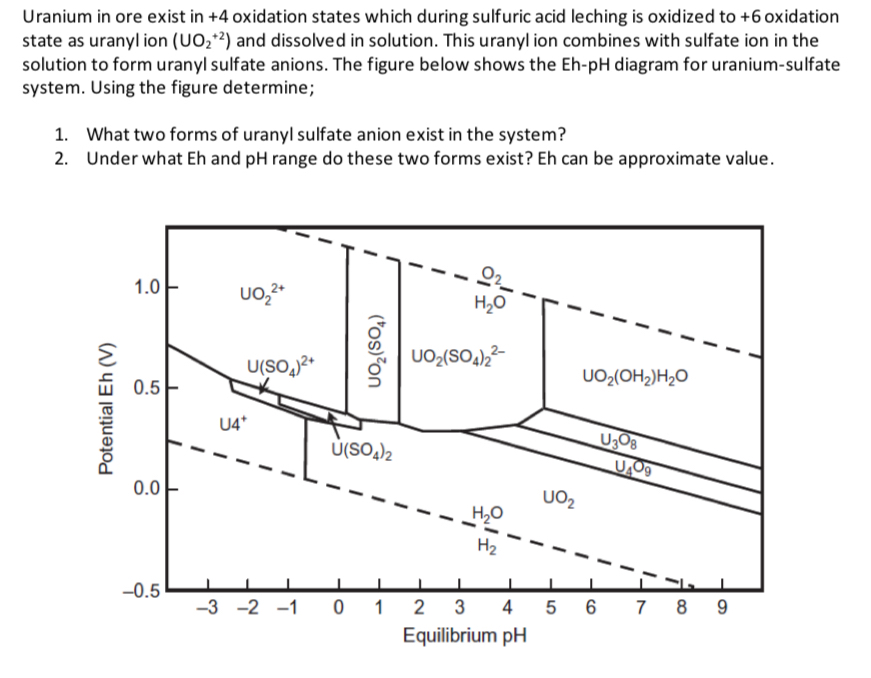
Uranium in ore exist in + 4 oxidation states which during sulfuric acid leching is oxidized to + 6 oxidation state as uranyl ion (
Uranium in ore exist in oxidation states which during sulfuric acid leching is oxidized to oxidation state as uranyl ion and dissolved in solution. This uranyl ion combines with sulfate ion in the solution to form uranyl sulfate anions. The figure below shows the Eh diagram for uraniumsulfate system. Using the figure determine;
What two forms of uranyl sulfate anion exist in the system?
Under what Eh and pH range do these two forms exist? Eh can be approximate value.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


