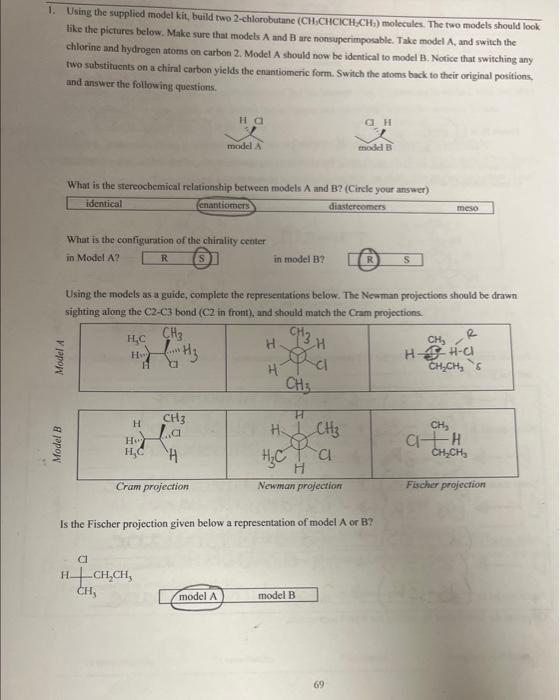
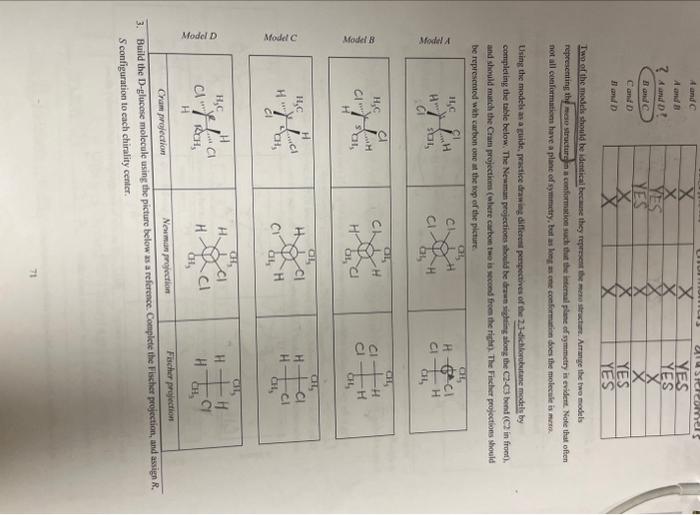
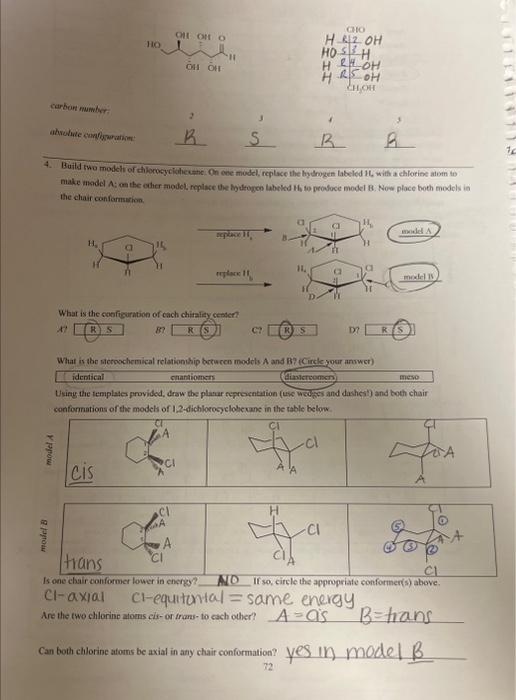
URGENT!! fill in all the blanks please
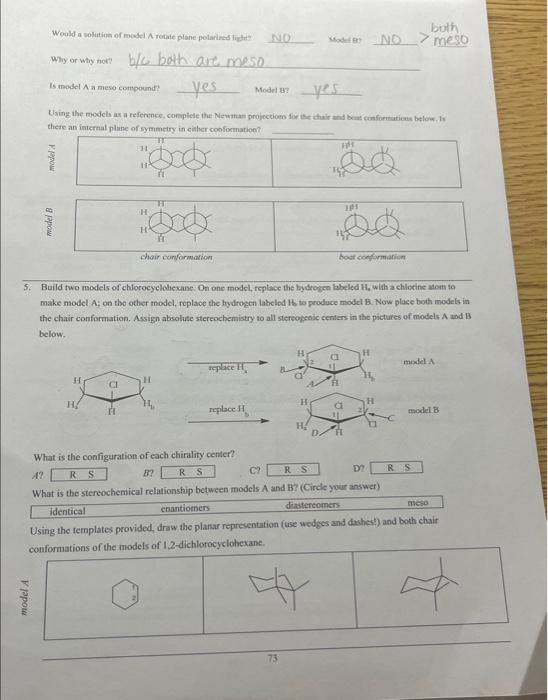
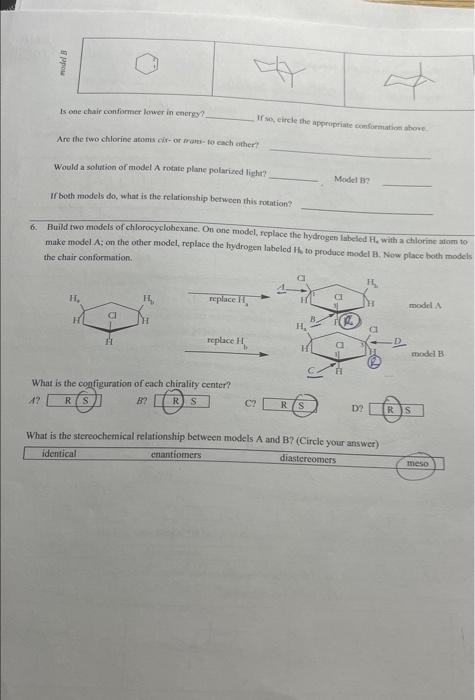
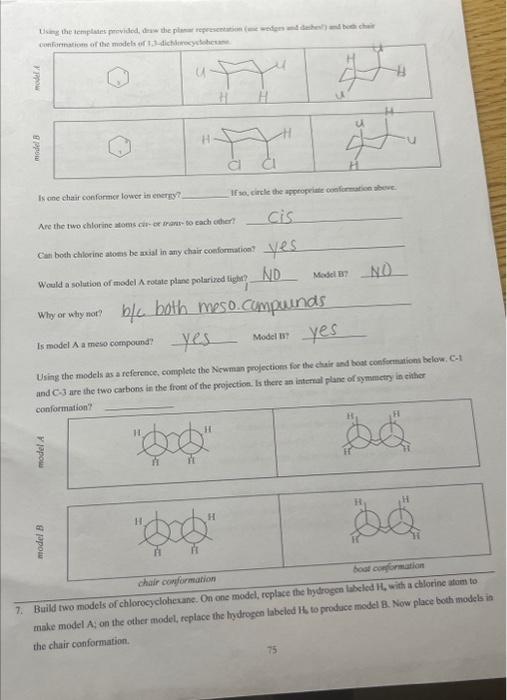
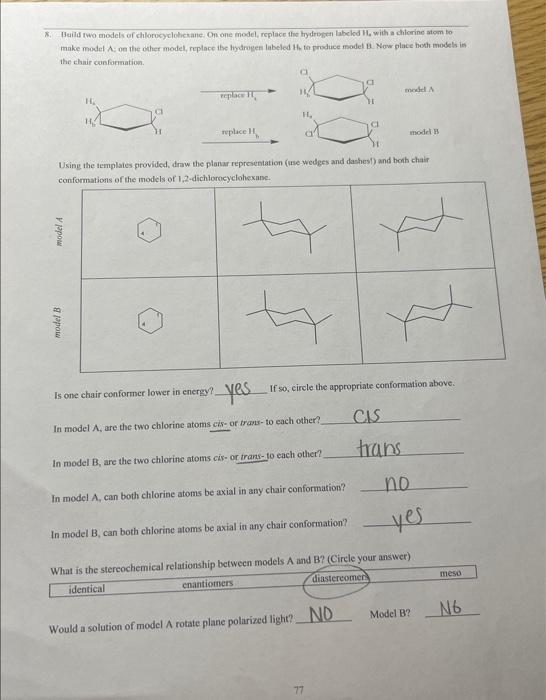
Would a solution of mogel A rotate plane poiarides fictre Miry or why noi? b/c both are meso Is model A a mieso compound? there an internal plane of symmetry in either confosmarion? 5. Build two models of chlorocyelohesane: On one model, foplace the bydrogen laheled ha with aghlorine atom to make model A; on the other inodel, replace the hydropen laheled 14 to produce model Ri. Now place both models in the chair conformation. Assign absolute stereochemistry to all sterecepenic centars in the pictures of miodels A and Bs below. What is the configuration of each chinality centcr? A? i? (E) D? What is the stcreochemierl relationship between models A and B? (Circle your answer) identical chantiofners arrese Using the remplates provided, draw the plartr representation (use wedges and dashes?) and both chair Is one chair conformar lonver in energy? If wo, elirele the appripriale cumformation ahove. Are the fwo chlorine atoms cir-or wane- fo cach wher? Would a solution of model A rotate plane polariced light? Movel B? If both models do, what is the relaticuship betwoen this rocation? 6. Build rwo models of chlorocyclohexane. On one model, feplace the hydrogen labeled H4 with a chlorine afom to make model A; on the other model, replace the hydrogen labeled Hi, to produce model B. Naw place hoth models the chair conformation. What is the cogfiguration of each chirality center? 17 BF R) S CPR D? Is ane chatit confarmer lovor in coctry? If yo, circle the apptogriate conformution abtue. Are the two chlorine atoens rut-or trant bo cach cadcrt Cati both chlorine abocas be axis in any chair conformation? Why or why mot? Is model A a mese coenpound? YeS Model n Y YeS Using the models as a reference, complete the Newman frojections for the chair and bost confremaions below, C-1 and C3 are the two carbons in the front of the projoction. Is there an internal plane of symansering in elitier conformation? chair camformation 7. Build two models of chlorocyclohesane. On one model, replace the hydrogen labeled H, with a chlorine atom to make model A; on the other model, replace the hydrogen labeled Hs to produce model B. Now place both models ia the chair conformation. What is the contigurariofi of each chiralify ccuter? (if) What is the sieteochermical relationship betwben models A and ig (Coincieyoor ampon) (idenrifal Usine the templates prosided, draw itse planar roprescetarion (wie uedges and dashes) and both chair canformations of the atodzls of 1 , 4-ticlabrucyclohesane Is ofe chavir conformer lower in encrey? If so, circle the appropeiate confocmation above: Are the two chiorine atoens cis-or frans-to cach ocher? Can both chlorine atouns be axial in any. chair coniormation?? Woald a soletion of modet A rotate plane polarined light? Model H? If both anodels do, what is the felationship between this rotation? 8. Heild two modelc of chlorocyelotickanc, On one model, replace the hydroben labelod H, with a chlorine atom to make model A: on the other model, replace its hydrogen labeled H ti produce model B. Now place hoth modets in the chair conformation. Using the templates provided, draw the planar representation (use-wedges and dachesi) and bonh chair In modelA, are the two chlorine atoms ejs of trats-to cach other? In model B, are the two chlorine atoms cis-or trans-to each other? B If so, circle the appropriate conformation above. In model A,can both chlorine atoms be axial in any chair conformation? Cis trans In model B, can both chlorine atoms be axial in any chair conformation? What is the stereochemical relationship between models A and B ? (Circle your answer) identical enantiomers diastereomen meso. Would a solution of model A rotate plane polarized light?? 1. Using the supplied model kit, build two 2-chlorobutane ( CH3CHClCHCH3) molecules. The two models should look like the pictures below. Make sure that models A and B are nonsuperimposable. Take model A, and switch the chlorine and hydrogen atoms ce carbon 2 . Model A should now be identical to model B. Notice that switching any two substituents on a chiral carbon yields the enantiomeric form. Switch the atoms back to their original positions. and answer the following questions. What is the stereochemical relationship between models A and B ? (Circle your answer) What is the configuration of the chimlify ecnter in Model A ? in model B ? Using the models as a guide, complete the representations below. The Nomman projections should be drawn. sighting along the C2C3 bond ( C2 in front), and should match the Cram projections. Is the Fischer projection given below a representation of model A or B? - Chlorobutane. On one model, replace the hydrogen labeled H w with a chlorine atom to make model A: on the ceher model, replace the frydrogen labeled H to produce model B. model. Sisosome Protan H, is Proton Ht is Now build fwo models of (S)-2-ehlorobuane, On one model, replaco the hydrogen labeled H, with a chilorine mom to make inodel C. on ibe other model, replace the hydrogen labeled H to produce model D. Provide names for each model by the R,S convention. (Note: according to IUPAC rules, always number from the end that gives RS ). madels ro compare: identical? enantiomers? diastercomers? Twn of the madels should be ifertical becuise they regrzect the mere structare. Arrange the hao models neprescriting ih a a conformation suct this the intermal plane of symmetry is evidert. Note that offen Using the models as a guide. prastice drawing diflerent propectives of the 23-dichlorobutane models by and should mutch the Cram projectioms (uhere carbon two is arcons froen the right). The Fincher projoctions thould be represenied whb carbon one at the top of the picture. 3. Build the D-glucase molecule using the picture below as a relerence: Compucve nise remse prownome assign R. S configuration to each chirality eenter. 4. Build two modeli of thloricycichecenc. On eve mudel, replace the hydopeen labeled 14, with a chlorine alom io make inodel A: on the edier model, roplace the hydrogon labeled H, to prodoce model 8 . Now place both models ia the chair confarmsirion. What is the cosfiguration of each chilalidy centen? it BR R(S) What is the ateroochemical relationihip betwces models A and B ? (Circleyour anower) identical chantiomens (Timertomen) meso Using the templates provided, draw the pharar eqpecentation (use weyses and dasheri) and both chair confonnations of the models of 1.2-dichlorosyelolecane in the table below Is one chair conformer lower in encrig? KD. If so, circle the appropriate conformer(s) above. cl-axial cl-equitcriat=sanne energu Are the two chlorine afoens cir-or frans- to each other? Can both chlorine atoms be axial in any chair conformation