Answered step by step
Verified Expert Solution
Question
1 Approved Answer
use matlab 2. The equation CP=a+bT+cT2+dT3 is an empirical polynomial that describes the behavior of the heat capacity CP as a function of temperature in
use matlab 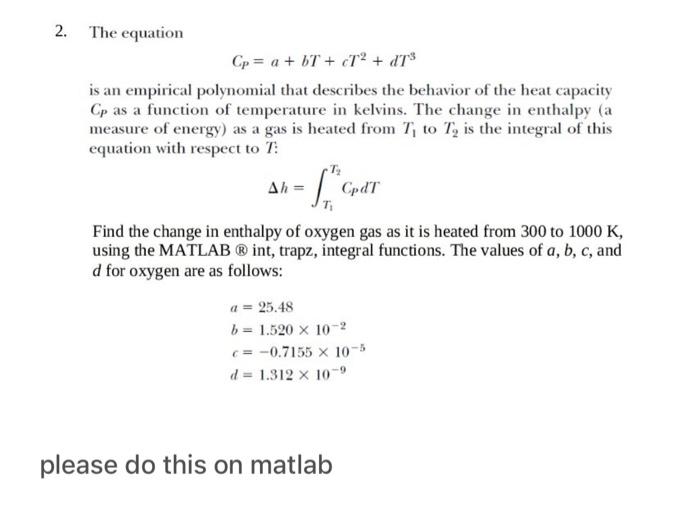
2. The equation CP=a+bT+cT2+dT3 is an empirical polynomial that describes the behavior of the heat capacity CP as a function of temperature in kelvins. The change in enthalpy (a measure of energy) as a gas is heated from T1 to T2 is the integral of this equation with respect to T : h=T1T2CpdT Find the change in enthalpy of oxygen gas as it is heated from 300 to 1000K, using the MATLAB int, trapz, integral functions. The values of a,b,c, and d for oxygen are as follows: a=25.48b=1.520102c=0.7155105d=1.312109 please do this on matlab 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


