Question: USE THE DATA FROM THE PHASE DIAGRAM AND TABLE UNDERNEATH IT ZOOM in On Image . E F F Pressure 00 B G A Temperature
USE THE DATA FROM THE PHASE DIAGRAM AND TABLE UNDERNEATH IT
ZOOM in On Image 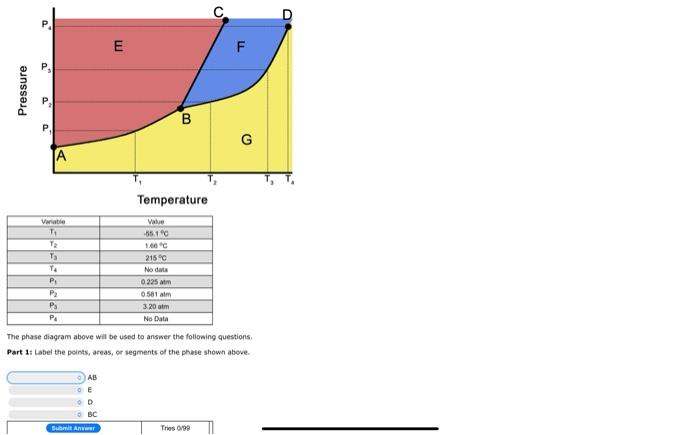
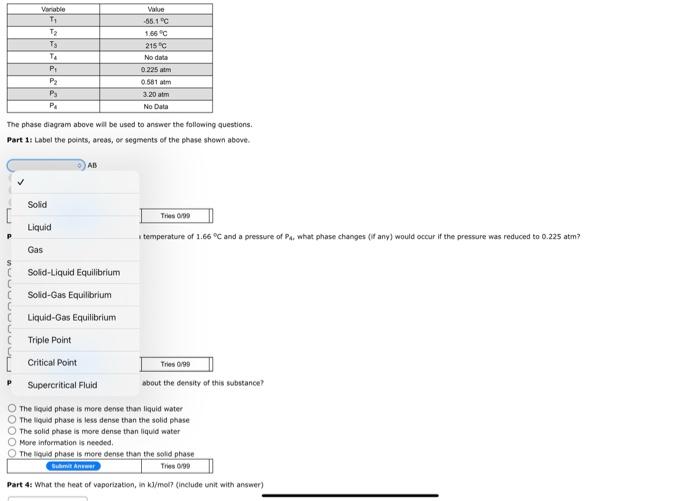

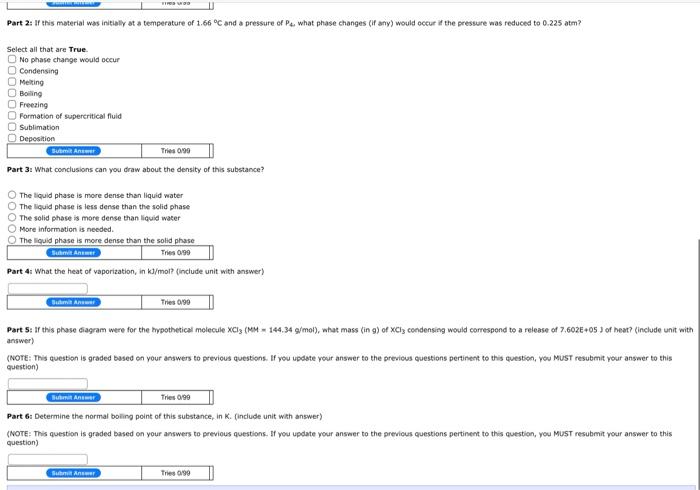
. E F F Pressure 00 B G A Temperature Variatie -12----1 Value -55.10 1.00 215C No data 0225 am 0.581 am 3:20 am No Data PS PA The phase diagram above will be used to answer the folowing questions, Part 1: Label the points, areas, or segments of the phase shown above AB E D BC Bumi An Tries/ Vanable TI PESHE Value -35.1 C 1.66C 215C No data 0.225 atm 0.581 am 3.20 am No Data P2 The phase diagram above will be used to answer the following questions. Part 1: Label the points, areas, or segments of the phase shown above. AB Solid [ Liquid This 6700 temperature of 1.66C and a pressure of what phase changes (if any) would occur if the pressure was reduced to 0.225 atm P Gas S Solid-Liquid Equilibrium C Solid-Gas Equilibrium Liquid-Gas Equilibrium c C c Triple Point [ Critical Point Tries 0/99 about the density of this substance? P Supercritical Fluid The liquid phase is more dense than liquid water The liquid phase is less dense than the solid phase The solid phase is more dense than liquid water More information is needed The liquid phase is more dense than the solid phase Bu Ant Tries0/90 Part 4: What the heat of vaporization, in k/mol (include unit with answer) BC But An Tries 0/90 Part 2: If this material was initially at a temperature of 1.66C and a pressure of Pa what phase changes (if any) would occur if the pressure was reduced to 0.225 atm? Select all that are True No phase change would occur Condensing Melting Bolig Freezing Formation of supercritical fluid Sublimation Deposition BAMI Time 0.00 Part 3: What conclusions can you draw about the density of this substance? The liquid phase is more dense than liquid water The liquid phase is less dense than the solid phase The solid phase is more dense than liquid water More information is needed The found phase is more dense than the solid phase B Anem Tres 000 Part 4: What the heat of vaporization, in k/mol? (include unit with answer) Baterie Anom Tries 6790 Part Si If this phase diagram were for the hypothetical molecule XCl, {MM 144.34 g/mol), what mass (in g) of XCl, condensing would correspond to a release of 7.6026+05 3 of heat? (include unit with answer (NOTE: This question is graded based on your answers to previous questions. If you update your answer to the previous questions pertinent to this question, you MUST resubmit your answer to this question) Sui Aneer Tries 0:00 Part 6: Determine the normal boiling point of this substance, in K (include unit with answer) (NOTE: This question is graded based on your answers to previous questions. If you update your answer to the previous questions pertinent to this question, you MUST resubmit your answer to this question) HRS Part 2: If this material was initially at a temperature of 1.66 C and a pressure of Pa, what phase changes (if any) would occur if the pressure was reduced to 0.225 atm? Select all that are True No phase change would occur Condensing Melting Boiling Freezing Formation of supercritical fluid Sublimation Deposition Buat Ant Tries 0:00 Part 3: What conclusions can you draw about the density of this substance? The liquid phase is more dense than liquid water The liquid phase is less dense than the solid phase The solid phase is more dense than liquid water More Information is needed. The liquid phase is more dense than the solid phase Sant Anime Tres 0/99 Part 4: What the heat of vaporization, in kl/mol? (include unit with answer) Batma Anwar Tries 0:00 Part 5: If this phase diagram were for the hypothetical molecule XCl3 (1M 144.34 g/mol), what mass (in g) of XCi3 condensing would correspond to a release of 7.602E+05 ) of heat? (include unit with answer) (NOTE: This question is graded based on your answers to previous questions. If you update your answer to the previous questions pertinent to this question, YOU MUST resubmit your answer to this question) Tries 0/90 Buhnit ARE Part 6: Determine the normal boiling point of this substance, in K. (include unit with answer) (NOTE: This question is graded based on your answers to previous questions. If you update your answer to the previous questions pertinent to this question, you MUST resubmit your answer to this question) Suht Answer Trio 090
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


