Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Use the References to A reaction is performed to study the reaction of UO_(2)^(+) with hydrogen ion 2UO_(2)^(+)+4H^(+)->U^(4+)+UO_(2)^(2+)+2H_(2)O The following reaction rate data was
Use the References to\ A reaction is performed to study the reaction of
UO_(2)^(+)with hydrogen ion\
2UO_(2)^(+)+4H^(+)->U^(4+)+UO_(2)^(2+)+2H_(2)O\ The following reaction rate data was obtained in four separate experiments.\ \\\\table[[Experiment,
[UO_(2)^(+)]_(0),M,
[H^(+)]_(0),M,Initial Rate,
Ms^(-) 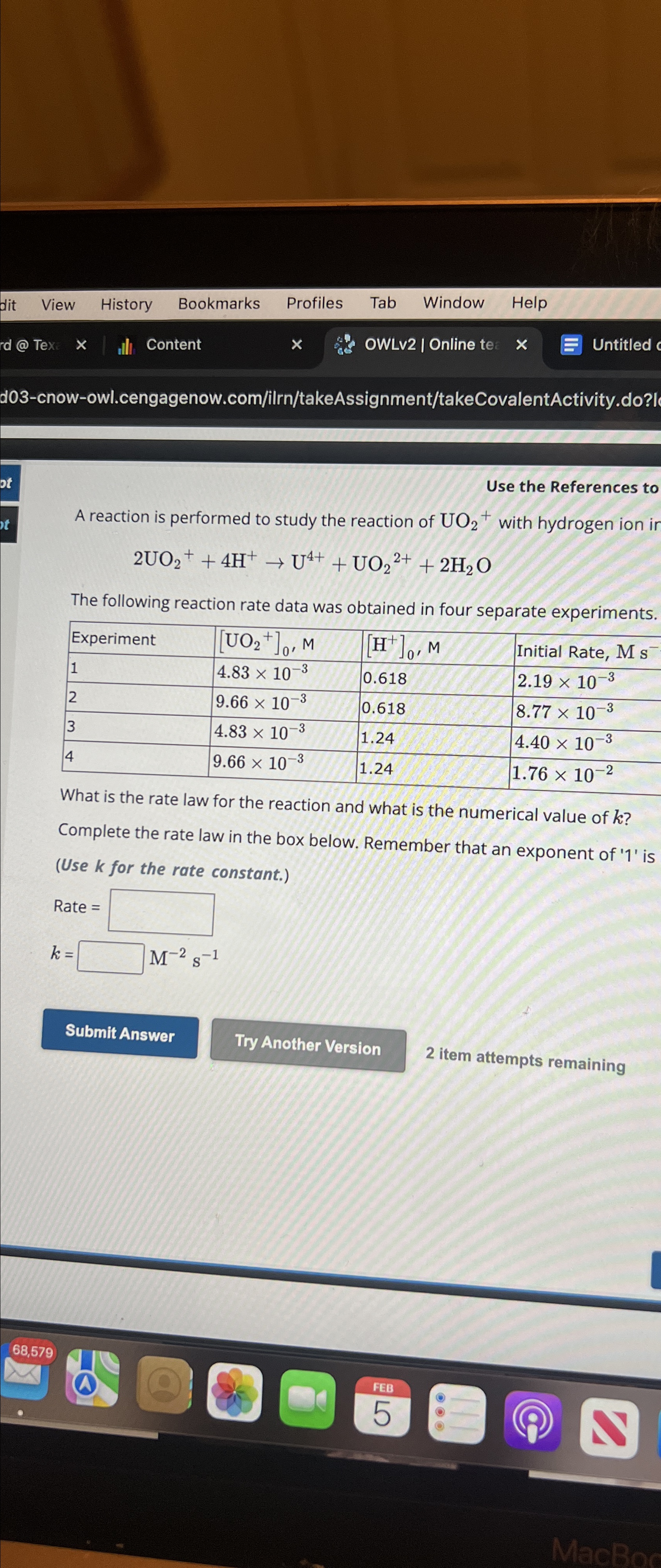 A reaction is performed to study the reaction of UO2+with hydrogen ion ir 2UO2++4H+U4++UO22++2H2O The following reaction rate data was obtained in four separate experiments. viratis the rate law for the reaction and what is the numerical value of k ? Complete the rate law in the box below. Remember that an exponent of ' 1 ' is (Use k for the rate constant.)
A reaction is performed to study the reaction of UO2+with hydrogen ion ir 2UO2++4H+U4++UO22++2H2O The following reaction rate data was obtained in four separate experiments. viratis the rate law for the reaction and what is the numerical value of k ? Complete the rate law in the box below. Remember that an exponent of ' 1 ' is (Use k for the rate constant.)
Use the References to\ A reaction is performed to study the reaction of
UO_(2)^(+)with hydrogen ion\
2UO_(2)^(+)+4H^(+)->U^(4+)+UO_(2)^(2+)+2H_(2)O\ The following reaction rate data was obtained in four separate experiments.\ \\\\table[[Experiment,
[UO_(2)^(+)]_(0),M,
[H^(+)]_(0),M,Initial Rate,
Ms^(-) 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


