Question
Use what you have learned in inorganic chem to answer these questions! 1. There were two main goals in inorganic chem that I have learned.
Use what you have learned in inorganic chem to answer these questions!
1. There were two main goals in inorganic chem that I have learned. The first was to differentiate metals from nonmetals. The second was to consider how materials respond on the atomic level to applied fields and how modifying chemical structure influences extrinsic properties, particularly conductivity and interactions with light.
a) A fundamental physical property of atoms is size. How does size influence whether an element is classified as a metal or nonmetal?
b) While electrostatic models can explain many properties of molecules, quantum mechanical models provide more extensive descriptions. With respect to solids, band theory can explain many properties. For elemental solids, draw a representative band diagram for a metal and one for a nonmetal.
c) How does atomic size play a role in the two band diagrams drawn above?
d) Band diagrams describe the electronic structure of elemental solids. Molecular structure and electronic structure are intertwined. Describe the fundamental molecular structures of metals and of one representative nonmetal solid.
e) Based on the above questions, explain why electrical conductivity varies by more than 20 orders of magnitude for elemental solids.
f) Based on the above questions, explain how the visual appearance differs for metals vs. nonmetal solids.
g) Describe how electrical conductivity varies with temperature for metals and nonmetal solids.
2. Briefly explain how an extrinsic semiconductor enhances the inherent electrical conductivity of an intrinsic semiconductor such as silicon.
3. Quantum dotsanoparticles have many current uses. One important property is a tunable emission as a function of size. Explain the basis for this effect.
4. In the history of coordination chemistry, [Co(NH3)5Cl]Cl2 is a very important molecule in terms of beginning to understand the structures of metal-ligand systems. Describe the types of bonding in this molecule.
5. Explain the fundamental basis for why coordinate covalent bonds form.
6. For the following compounds, (i) provide the oxidation state and (ii) number of d electrons for the central metal, then (iii) draw one isomer of the compound and finally (iv) provide the full name of that isomer.
a) [Mn(ox)2BrCl] 3-
b) [Co(H2O)3(NH3)3]
7. H2NCH2CH2NHCH2CH2NH2 (det) is a tridentate ligand. Write a balanced equation for the reaction that occurs when Ni(H2O)6 2+ is exposed to excess det and suggest the driving force for this reaction.
8. CdS is often used in the photometers of cameras to measure the available visible light. Suggest a related material that could be used similarly for ultraviolet photography for example of kingfishers. Explain your choice.
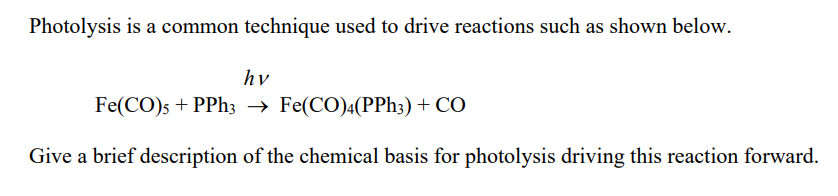
9.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


