Answered step by step
Verified Expert Solution
Question
1 Approved Answer
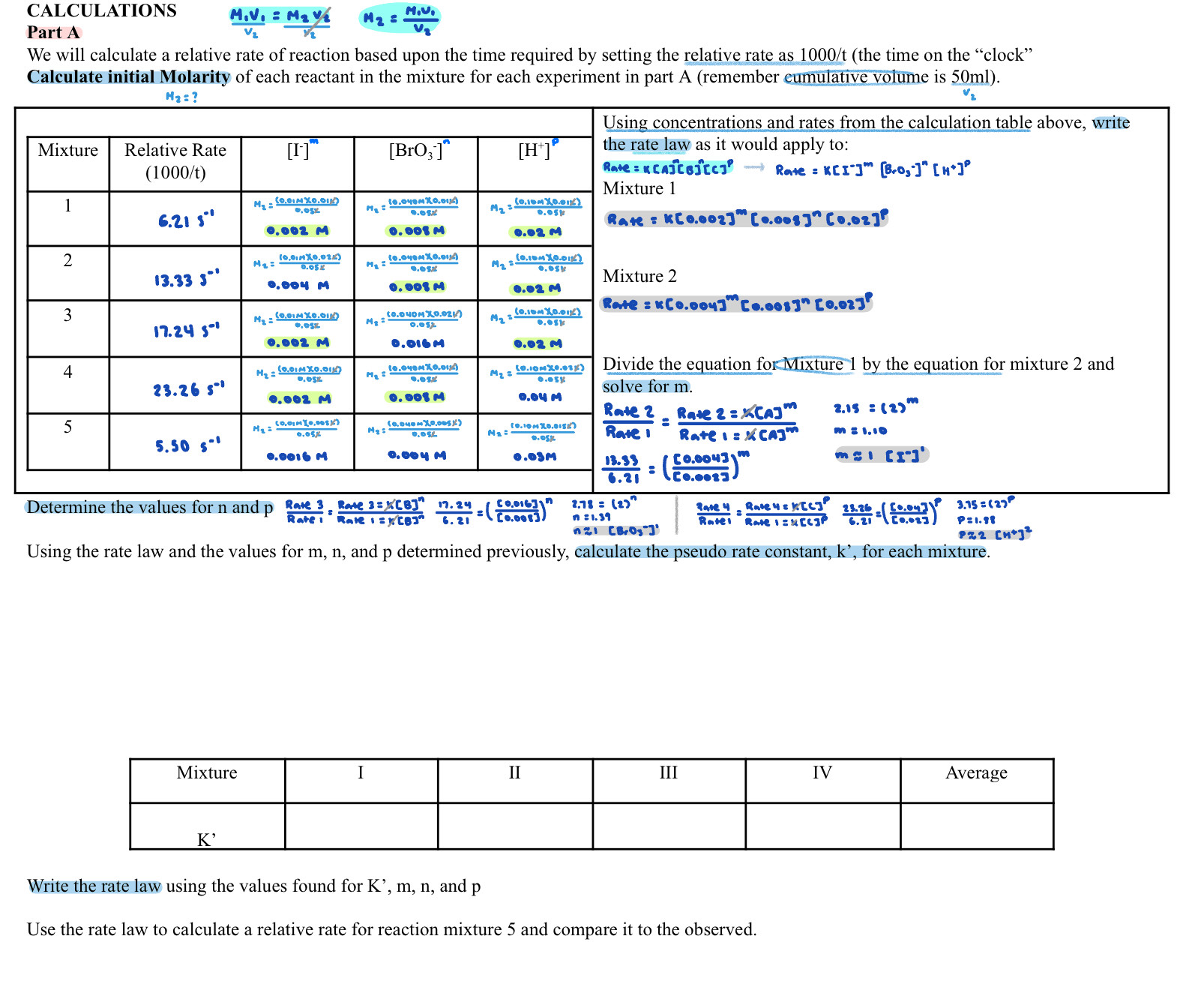
using the rate law and the values for m , n , and p determined CALCULATIONS Part A M 1 V 1 V 2 =
using the rate law and the values for m n and p determined CALCULATIONS
Part A
We will calculate a relative rate of reaction based upon the time required by setting the relative rate as t the time on the "clock"
Calculate initial Molarity of each reactant in the mixture for each experiment in part A remember eumulative volume is
Using concentrations and rates from the calculation table above, write
the rate law as it would apply to:
Rate Rate
Mixture
Rake
Mixture
Rate
Divide the equation formixture by the equation for mixture and
solve for
Using the rate law and the values for and determined previously, calculate the pseudo rate constant, for each mixture.
Write the rate law using the values found for and
Use the rate law to calculate a relative rate for reaction mixture and compare it to the observed.previsously, calculate the pseudo rate constant, K for each mixture
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


